Salvinorin A

Attempts to depict the visual hallucinations induced by S. divinorum through painting (illustrations from deviantart.com).
Salvinorin A is the most potent of all known naturally occurring hallucinogens. It is ten times stronger than psilocybin and nearly as potent as the semi-synthetic psychedelic LSD. It is found in the leaves of the Sage of the Diviners (Salvia divinorum), a very rare plant that grows in remote regions of Mexico at elevations of 750–1500 meters above sea level. This plant has long been used by the Mazatec Indians for ritualistic and medicinal purposes. The Mazatecs associate the Sage of the Diviners with the image of the Virgin Mary, which is why its local names, such as herb-of-the-virgin, hierba de Maria, and Maria Pastora, translate to "herb of the Virgin Mary" or "leaves of Mary the Shepherdess."[4]
Although the ritual use of tea made from the "herb of the Virgin Mary" was first described by Swedish anthropologist Jean Basset Johnson in 1939, the first samples of this mysterious plant were not obtained until 1962 by the famous American millionaire and ethnobotanist R. Gordon Wasson.[1]
In 1962, Wasson became the first Westerner to experience the effects of salvinorin A after a Mazatec shaman offered him juice extracted from 34 leaves of the sacred plant. According to Wasson, the effects of salvinorin A were similar to those of psilocybin, but shorter in duration and less intense. The visual hallucinations he experienced resembled "dancing colors in elaborate, three-dimensional designs".[4]
Accompanying Wasson on the expedition was Albert Hofmann, the renowned Swiss pharmacologist who first synthesized and tested LSD. This time, he aimed to isolate the active hallucinogenic component of Salvia, but he was unsuccessful—the alcohol extract of Salvia lost its magical potency during the journey back to Switzerland.
It wasn't until 1982 that Alfredo Ortega successfully isolated Salvinorin A in its pure form[24], and in 1993, Daniel Siebert, after smoking Salvia divinorum leaves, provided a detailed description of its remarkably potent hallucinogenic effects.[11]
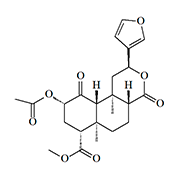
Salvinorin A
Mechanism of Action. In 2002, it was discovered that Salvinorin A is a potent and selective agonist of the opioid κ-receptors.[6] By modifying the Salvinorin A molecule, researchers attempted to determine which part of it was responsible for its remarkable hallucinogenic activity. Over the following decade, more than a hundred Salvinorin derivatives were synthesized and studied. It was found that the furan ring and the ester bond at the 2-position are the most critical for its psychotomimetic effects.[6] Additionally, it was determined that the methoxymethyl group at the 2-position increases κ-activity in vitro by 7 times.[7]
Activity with Different Routes of Administration. The original indigenous preparation method involved thoroughly mashing the leaves in water and then consuming the resulting beverage. When taken orally, the Salvia solution has a very mild effect on the psyche, which is why the Mazatecs only used it when Psilocybe mushrooms or Morning Glory seeds (Ipomoea violacea) were unavailable. The shamans themselves consider it the weakest of the three hallucinogens they use.[4]
It is unclear why the Mazatecs did not use more effective methods of consumption, such as smoking or chewing the leaves. Interestingly, the latter method was discovered relatively recently, in 1993, by a group of enthusiasts in California. When chewing, to enhance the absorption of salvinorin A through the mucous membranes of the mouth, the chewed leaves are not swallowed but are placed as close to the cheek as possible.[12] Salvinorin A is easily absorbed through mucous membranes. A noticeable effect occurs after 10 minutes of chewing 8–10 large (about 3 grams) fresh leaves. At the same time, simply swallowing the same amount of leaves does not produce any noticeable effects. Even 10 mg of pure salvinorin A placed in a gelatin capsule and swallowed does not cause significant perceptual changes, as salvinorin A is rapidly broken down in the gastrointestinal tract.[11] With sublingual (under the tongue) administration of salvinorin A solutions in dimethyl sulfoxide (DMSO) or acetone, only 100–250 micrograms are needed.[12]
Salvinorin A melts at around 240°C and sublimates readily. When inhaling its vapors, a pronounced psychotomimetic effect develops at doses of 200–500 µg. Doses of 500–1000 µg and above are dangerous, as the person completely loses touch with reality and cannot control their behavior.[2]
Toxicity. Cats can tolerate a dose 635 times higher than the psychotomimetic dose for humans without any adverse effects. When salvinorin A emulsion was administered orally to mice, death did not occur even at a dose of 1 gram per kilogram, which is 10,000 times higher than the effective dose for humans. However, this low toxicity may be due to the poor absorption of salvinorin A in the stomach. When administered intraperitoneally, the median lethal dose (LD50) for mice is 340 mg/kg.[18] which is almost twice that of acetylsalicylic acid.[19]
Extraction: Salvinorin A is extracted from the leaves of S. divinorum using organic solvents (chloroform, acetone, methanol) at room temperature[18]. The salvinorin A content in dried leaves varies widely, ranging from 2 to 7.8 grams per kilogram[3,23]. The dry leaf mass is about 13% of the fresh leaf mass. Siebert noted that leaves harvested in the summer contain twice as many active compounds as those collected in the winter[1].
In 2007, J. Scheerer achieved the total chemical synthesis of salvinorin A. The 29-step synthesis yielded the compound with an overall efficiency of 0.8%[21].
Symptoms of Poisoning. When inhaled, salvinorin A vapors act almost immediately, with symptoms intensifying within 10–20 seconds, peaking at around 30 seconds, remaining at this level for 3–10 minutes, and gradually subsiding after 10–20 minutes[1].
Under the influence of low doses of salvinorin A, only mild visual distortions occur, accompanied by a sense of relaxation and a philosophical or mystical mood.




Attempts to depict the visual hallucinations induced by S. divinorum through painting (illustrations from deviantart.com).
At moderate doses, Salvia divinorum enhances emotions, sharpens perception of the surroundings, creates a feeling of lightness in the limbs, and can cause dizziness and nausea. As the dose increases, vivid visual hallucinations and a disruption of the "body schema" appear. The hallucinations are complex and very realistic, with the person becoming fully immersed in events of another reality, sometimes living an entire life in other worlds within just a few hours. Often, there is a sensation of transforming into living beings, plants, or inanimate objects. Other effects include a loss of personal identity, a sense of being in two places at once, illusions of movement, and uncontrollable laughter.[11]
Siebert describes the behavior of people who have taken a large dose of salvinorin A as follows:
"When the dose goes above 500 to 1000 mcg the effects can be very alarming. I have seen people get up and lunge around the room, falling over furniture, babbling incomprehensible nonsense and knocking their heads into walls. Several people have tried to wander out of the house. When the experience is over, they have no memory of any of this. In fact, they usually remember very different events. To an outside observer, people in this condition have a blank look in their eyes as if no one is present. It is also common for people to have a facial expression which is probably best described as being like that of a frightened animal."[2]
The unpredictability of its effects often deters many experimenters from using salvinorin A again. For most people, the experience is so unpleasant that the term "salvia effect" has been coined — where the end of the trip is more pleasurable than the trip itself.
Salvinorin A did not have a significant effect on blood pressure or heart rate,[20] but at high doses, it may cause an increase in body temperature.[12]
Salvininorin A does not appear to be a drug that would lead to addiction, and at least until 1996, there were no documented cases of drug dependence.[10] According to a survey conducted in 2004, only 1.2% of Salvia users reported strong cravings, and even in those cases, withdrawal symptoms were not observed upon cessation.[9]
«Symmetry»
Symmetry (2-EtOMe-Sal B) is the ethoxymethyl ether of salvinorin B, first synthesized by T. Munro et al. in 2008[15], and a year later, this substance was tested on four volunteers[16].
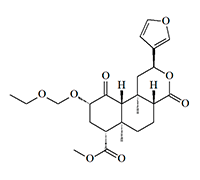
«Symmetry»
Ethoxymethyl ether of Salvininorin B
The psychotomimetic "Symmetry" got its name from its ability to induce visual hallucinations in the form of fantastically symmetrical geometric figures. The threshold dose for "Symmetry" is 10–50 µg, with pronounced effects occurring at 150–300 µg, which is several times lower than that required for salvinorin A.




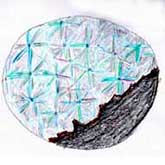

Psychotomimetics often cause visual hallucinations in the form of geometric shapes (form constants): LSD — tunnels or funnels (the first two images on the left), marijuana — honeycomb patterns (third from the left)[17]. "Symmetry" is characterized by geometric visual patterns resembling spokes or snowflakes, as attempted by an experiment participant in the image on the far right, or as depicted in the artwork by the artist known as SalviaDroid in the page header.
The psychotomimetic effects of "Symmetry" are felt almost instantly, within seconds of inhalation, and quickly subside within 5 minutes. A dose of 100–200 µg causes geometric distortions, both with eyes open and closed, palinopsia—a visual perception disorder where an image persists or reappears after the object has disappeared from view—and a sense of impending danger. Higher doses lead to confusion, derealization, and vivid geometric hallucinations. One subject rated a 400 µg dose as "++++" on the A. Shulgin scale, which includes substances that "induce a state of divine enlightenment, mystical experiences, and a sense of connection with the inner and outer universe".[16]
Salvinorin A as a Potential Incapacitant
The chemical structure of salvinorin A was discovered after the official end of the U.S. chemical warfare program, and there are no mentions of its use as an incapacitant, although the idea has been discussed in "non-specialized" forums.[25] However, kappa-opioid receptor agonists with similar mechanisms of action were studied by the U.S. in the 1960s and 70s as potential incapacitants.
Psychomotor agitation, which occurs in 5% of cases,[1] can be mitigated by the concurrent use of neuroleptics or sedatives. The combination of a hallucinogenic kappa-agonist and a neuroleptic was studied as a potential incapacitating agent in the United States. In addition to neuroleptics, salvinorin A enhances the effects of other substances that depress the central nervous system. In a small series of experiments on rats, the administration of sedative doses of a tranquilizer (Diazepam) or an alpha-2 adrenergic agonist (Medetomidine) in combination with salvinorin A produced immediate unconsciousness and rapid general anesthesia.[14]
Salvinorin A has two unique qualities for an incapacitant — almost instantaneous action and a short duration of effect. Positron emission tomography has shown that the peak concentration of salvinorin A in the brain is reached by the 40th second, which is nearly 10 times faster than cocaine, and by 16 minutes, the psychotomimetic is virtually undetectable.[22]. Salvinorin is nearly as potent as LSD and BZ, is active when inhaled, has low toxicity, and lacks serious side effects.
Salvinorin A sublimates easily and can be used in vapor form, with its solutions also being applicable as an aerosol. Dried salvia leaves retain their activity for an extended period[12], indicating that salvinorin is chemically stable.
Salvinorin A is difficult and expensive to produce synthetically, but many biologically active compounds derived from plant sources have been studied as potential chemical warfare agents (ricin, veratrine, aconitine, LSD, nicotine, and others). Some are still used today as riot control agents and in self-defense products (capsaicin, piperine, gingerol, allyl isothiocyanate).