Chemical Agent PAVA
Synonyms: Nonivamide, ВАПЕКИС, Capsaicin II, Pseudocapsaicin, PAVA spray, N-Van, VP, PV, VN.
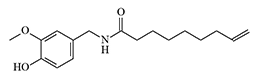
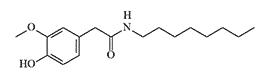
Vanillylamide of pelargonic acid
History of Development and Use In 1919, E.K. Nelson synthesized a series of vanillylamides of saturated carboxylic acids, structurally similar to the active component of red pepper — capsaicin, and with a pronounced burning taste. The most intense irritating effect was found in the vanillylamide of pelargonic (nonanoic) acid.[8] This substance would later receive the abbreviation PAVA — Pelargonic Acid VanillylAmide.
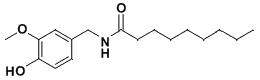
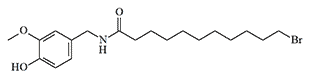
In 1921, E. Ott and K. Zimmermann first synthesized vanillylamide of undecylenic acid in crystalline form.[24] This irritant was considered a promising chemical warfare agent due to the fact that undecylenic acid required for its synthesis could be produced from relatively inexpensive castor oil. This substance exhibited an even more intense burning sensation than PAVA.[23] Nelson wrote, that "vanillyl undecoylamide, affects the throat rather than the tongue. Its powder is more irritating to the nose and throat, however, causing violent coughing and sneezing".[8]
In the 1920s and 1930s, a series of vanillylamide derivatives of saturated and unsaturated acids were studied as potential chemical warfare agents in the United States, the United Kingdom, Australia, and the USSR. Military chemists discussed the use of these agents as standalone agents or in combination with other irritants: in thermal generators with adamsite and chloroacetophenone, or in liquid mixtures with chloropicrin or ethyl bromoacetate.[13,19]
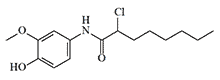
In 1934, the British military chemist A. H. Ford-Moore synthesized and studied the activity of 16 vanillylamides of saturated fatty acids.[22] During World War II, the United Kingdom and the United States, fearing a potential shortage of arsenic for the production of standard irritant agents, continued research on vanillylamides of undecylenic, nonanoic, and mandelic acids.[25,30] In 1942, trials on volunteers were conducted in the United Kingdom at the Chemical Defence Experimental Station (CDES) on a mixture of PAVA and diphenylchloroarsine.[30]
In the second half of the 20th century, prominent toxicologists such as Y. Alarie (1990).[30] and B. Ballantyne (1999).[17] studied the irritant effects of vanillylamides. In the 1960s, Y. Alarie developed a testing method for chemical irritants on animals at Edgewood Arsenal and was the first to use it for screening potential riot control agents, including vanillylamide derivatives. B. Ballantyne researched the activity of N-phenylamides and guaiacylamides of carboxylic acids and their 2-chloro derivatives, such as HMPC, which exhibited irritant effects on animal eyes and respiratory tracts comparable to PAVA.[17] There is no information available on human testing for irritants of these groups.
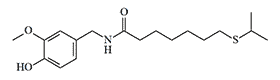
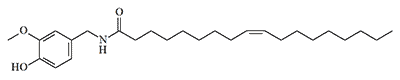
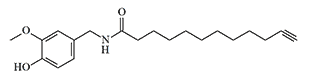
| Formula or Name | RD50 | Toxicity (mice) |
|---|---|---|
| Agent CR | 2,00 | LD50 — 37,2 mg/kg, IV LD50 — 242 mg/kg, IP |
 |
6,80 | LD50 — 5,6 mg/kg, IV LD50 — 33 mg/kg, IP |
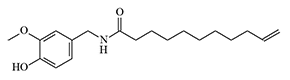
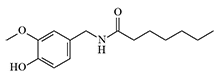
| PAVA | 8,20 | LD50 — 8 mg/kg, IP |
 |
9,00 | LD50 — 3,2 mg/kg, IV |
| Agent CS | 9,16 | LD50 — 47,7 mg/kg, IV LD50 — 32 mg/kg, IP |
 |
10,20 | LD50 — 350 mg/kg, IP |
| Capsaicin | 10,35 | LD50 — 0,4 mg/kg, IV |
 |
11,20 | LDmin — 50 mg/kg, IP |
 |
22,80 | LDmin — 50 mg/kg, IP |
 |
23,00 | No data |
 |
26,20 | No data |
 |
27,80 | No data |
 |
32,50 | LDmin — 50 mg/kg, IP |
 |
36,00 | No data |
LD50 — Median lethal dose. Route of administration: IV — intravenous, IP — intraperitoneal
RD50 – the concentration of a substance in air (in mg/m3) that reduces the breathing rate in mice by 50%.
Inhalation toxicity data are from Y. Alarie (1990).[30] Parenteral toxicity data are sourced from ToxNet.
In 1974, a patent was issued in Japan (JP4935198) for the use of the synthetic capsaicinoid PAVA in self-defense products as a safer alternative to "pepper sprays".[5]
In 1995–1996, the Swiss police funded toxicological studies of PAVA.[28] Later, tear gas canisters containing PAVA were approved for use by the police in Switzerland and Austria, though they were not widely adopted.[11]
In the late 1990s, the non-lethal weapons market saw the introduction of paintball systems such as PepperBall and FN 303, which were equipped with projectiles containing PAVA.

LIFELITE device and TCP pistol for firing Pepperball projectiles
In 2001, the Sussex police received the first batch of spray PAVA for testing, and over the next few years, police in two other British counties also adopted PAVA sprays.[11]
According to many self-defense product developers, PAVA offers significant advantages over OC, as it does not depend on the quality of plant raw materials and lacks ballast substances, making its effects more predictable. Comprehensive medical tests, conducted primarily in the United Kingdom, confirmed the effectiveness and safety of PAVA, after which it was adopted by police forces in the United Kingdom, Germany, Switzerland, Belgium, and the Netherlands.[21]
The U.S. Food and Drug Administration (FDA) approved the use of PAVA in the food industry to add a spicy flavor to meat products, soups, and even chewing gum.[9,11] PAVA is the primary active ingredient in the ointment "Finalgon," used for treating inflammatory diseases of the spine, joints, and muscles. PAVA is also included on the list of prohibited substances (doping) for horses.[14]
Physical properties: Solid substance with a characteristic odor, melting point of 54–56ºC, boiling point of 200–210ºC (at 0.05 mm Hg). Soluble in water, ethanol, and methyl chloride.
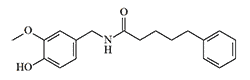
Synthesis: Although PAVA is often called "synthetic capsaicin", it belongs to natural compounds, as it has been found in trace amounts in chili peppers.[7] First synthesized by Nelson in 1919.[8]

Another early method for obtaining vanillylamides of unsaturated acids (Bradner & Sherrill, 1924) consists of two stages. Initially, the unsaturated acid amide reacts with formaldehyde, and then the resulting oxymethylamide is condensed with guaiacol:[27]
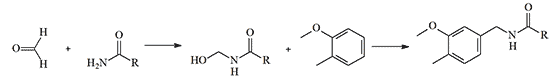
In particular, this method was used for the synthesis of such an irritant as vanillylundecenylamide.[20]
Toxicity: As an irritant, PAVA is more potent than CS but less irritating than CR and capsaicin.[6,8] The pungency of PAVA is 9,200,000 SHU, nearly half as strong as capsaicin, which has 16,000,000 SHU.[1] When PAVA concentration in spray is below 1%, it primarily induces lacrimation. At concentrations above 5%, it causes skin and upper respiratory tract irritation.
The first spray canisters provided to the Hertfordshire Police contained a 0.1% PAVA solution, as this concentration was determined to be safe even for people suffering from bronchial asthma. However, this concentration proved insufficient to fully incapacitate offenders, especially if they were under the influence of alcohol or drugs. Meanwhile, the Zurich Police, equipped with sprays containing 0.64% PAVA concentration, found them too powerful and dangerous. Eventually, a compromise solution was reached - a 0.3% PAVA solution, approximately the same concentration found in PAVA-based medicinal ointments.[16]
Unlike CS, PAVA causes only mild respiratory irritation but induces a more intense and prolonged pain response when directly applied to the eyes. Upon eye exposure, pain occurs almost immediately and lasts longer compared to the effects of CS.[11]
| Method | Toxicity | Ref |
|---|---|---|
| LD50 (mice, oral) mg/kg | 5110 | [32] |
| LD50 (mice, intraperitoneal) mg/kg | 8.0 | [12] |
| LC50 (mice, inhalation) mg/min·m3 | >864,000 | [32] |
PAVA is significantly more toxic than CR and CS when administered parenterally. However, according to the Swiss Ministry of Interior, PAVA is safer than other police irritants when inhaled (source not specified).[32]
Symptoms of poisoning and treatment of casualties: See Oleoresin capsicum (OC)
Use of PAVA Spray
PAVA is most commonly used in concentrations of 0.1–0.65% in alcohols: methanol, ethanol, isopropanol, or their aqueous solutions.[2] For example, UK police are equipped with canisters containing a 0.3% solution of PAVA in ethanol. The effective range of such a spray is up to 5 meters, and typically, a 0.5–1 second spray duration is sufficient to affect a target.[3,10] Outdoors, the effects usually dissipate within 15–20 minutes. It may also be used in combination with other irritants (CS, CN).
| IDC System | PepperBall Technologies, Inc | |||||
 |
 |
 |
 |
 |
 |
|
| RSG-1 | RSG-2 | RSG-3 | Curd`s Police | Anti-Attack | PAVA Pepper Spray | |
PAVA-based special equipment is manufactured by the following international companies: Carl Hoernecke Chemische (Germany), IDC Chemie Handels (Germany), IDC System (Switzerland), Civil Defence Supply (UK), PepperBall Technologies, Inc (USA).
FN303 Police Less-Lethal Launcher
Belgian company FN Herstal produces two types of paintball-style less-lethal weapons: the 15-round pneumatic automatic rifle FN 303 and the seven-round semi-automatic pistol FN 303P. These weapons use frangible PAVA-containing ammunition that breaks upon impact with the target.
 Model FN303 |
 Model FN 303P |
 Non-lethal projectile with PAVA |
The effective firing range is 20 meters for the FN 303P pistol and 50 meters for the rifle. The projectile weight is 8.5 grams.
The FN 303 was used by American infantry units and military police in Iraq. In 2013, this model was employed by Turkish police to suppress anti-government protests.[29]
At least one fatal incident has been associated with the FN 303. In 2004, during riots in Boston, a 21-year-old female student was fatally injured when a paintball projectile struck her eye.[26]
Pepperball Automatic Air-Powered Launcher
Pepperball is another type of less-lethal weapon. The name Pepperball comes from its projectiles - plastic balls containing the irritant PAVA (Capsaicin II). When hitting a person, the shell of such a ball breaks, releasing the irritant. Compressed gas (CO2) is used to propel the projectile.
The TAC 700 can fire automatically at a rate of up to 700 rounds per minute with an effective range of up to 60 meters.
 Model TAC-700 |
 Model SA-4 |
 Paintball rounds with PAVA |
PepperBall was successfully used by police during antiglobalization riots in Seattle (1999), against basketball fans, and during the "drunken riot" at the 2002 Salt Lake City Olympics. In 2004, the US Border Patrol purchased 14 PepperBall systems that use ammunition containing powdered PAVA or OC. This purchase followed three years of testing, during which this type of less-lethal weapon was used in more than 80 incidents along the US-Mexico border.[18] Besides the Border Patrol, PepperBall is used by the Federal Bureau of Prisons, Coast Guard, Immigration and Customs Enforcement, Sheriff's Departments, and many US police departments.
Other Synthetic Capsaicinoids
"Pepper Gas" (Pfeffergas, Vanillylheptoylamide.) is a black viscous liquid with a high boiling point (250ºC), causing sneezing and coughing in humans at concentrations of 50 mg/m3.

In the 1930s, US Chemical Service Lieutenant Colonel Byron C. Goss played a major role in the development and promotion of this chemical agent, considering Vanillylheptoylamide one of the most promising irritants.
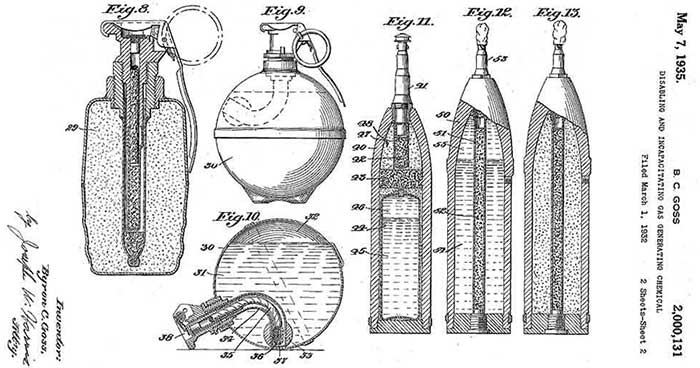
Various combat delivery systems for vanillylheptoylamide proposed by B. C. Goss (1935)
He developed liquid and powder formulations based on vanillylheptoylamide suitable for use in cartridges, hand grenades, and shells.[19]