Thebaine derivatives

Dexter Morgan, the protagonist of the TV series of the same name, immobilizes his victims with the drug M99, also known as Etorphine.
The semi-synthetic opioid analgesics of the thebaine group were first synthesized and studied by English chemist Kenneth Bentley in the early 1960s. In his honor, this group of substances is also called "Bentley compounds". The high activity of the new analgesics was discovered by accident. Once, during a morning tea party, one of the employees of K. Bentley decided to stir himself and his colleague's tea, lying nearby glass rod, which left traces of the derivative thebaine. Not a few moments later, both scientists found themselves on the floor in a semi-conscious state.[1] It was the harsh years of the Cold War and therefore, for the benefit of the Fatherland, it was decided to transfer the new substances, with incredible for that time activity, to the British Chemical Defense Experimental Establishment (CDEE), where work on new types of chemical weapons was carried out.[17]
Sources and preparation. The starting product for the synthesis of etorphine and related compounds is the alkaloid thebaine, which is obtained from the bracteate poppy (Papaver bracteatum). Unlike the opium poppy (Papaver somniferum), this species of poppy is virtually free of morphine and codeine. Despite the plant's lack of narcotic opioids, it is still illegal to grow in the United States because the thebaine it contains can be used to produce etorphine or its derivatives in two stages. Ethorphine approaches morphine and heroin[8] in its ability to induce euphoria and addiction.
As potential non-lethal chemical weapons, these substances were studied in military chemical laboratories in the United States (1967–1975)[2], United Kingdom (1961–1972), Switzerland (1976)[3], China (1982–)[10], the Republic of South Africa (1993)[5], and the Czech Republic (2005).[6]
 |
 |
 |
 |
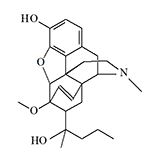
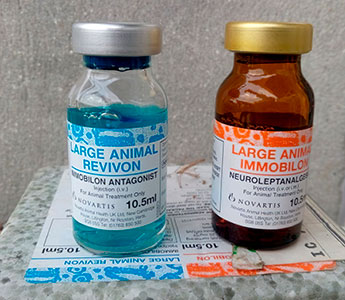
| Etorphine (M99), Captivon© |
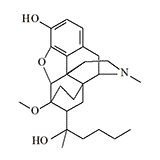
RX 140M (M–140) |
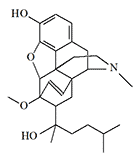
Dihydroetorphine (DHE, DHE, DHM99) |
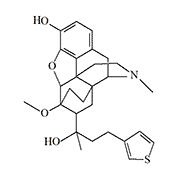
TH-030418 (TH–002) |
Etorphine (Etorphine, Immoblion, M99®). The best-known member of this group is etorphine, which, even by conservative estimates, is 500 times more potent than morphine and 250 times more potent than heroin.[8] Because of this high activity, etorphine is best suited for immobilizing large wild animals. Its effects are so strong that just 1 mg of the drug is enough to put a rhinoceros weighing 2 tons to sleep, while 4 mg can knock down a 5-ton elephant. Veterinarians also appreciate etorphine for its quick action, it starts to act 2–4 min after administration, reaching its maximum in 15 min. The duration of immobilization does not exceed 1 hour.
In general, etorphine was no worse than other common analgesics of those years, but its use for immobilization of elephants and rhinos caused irreparable damage to etorphine's reputation — pharmaceutical companies were in no hurry to market the drug known as "elephant tranquilizer". In addition, the WHO placed etorphine on the list of narcotic substances subject to the strictest accounting and control.[14]
In humans, when administered intramuscularly, 0.02 mg of etorphine produces the same euphoria as 10 mg of morphine or 5 mg of heroin. The analgesic dose of etorphine is several times higher, about 0.07 mg. The researchers noted that "the extremely rapid onset of etorphine was striking and effects were apparent within 5 min. A number of subjects reported effects before the completion of the subcutaneous injection".[8]
 |
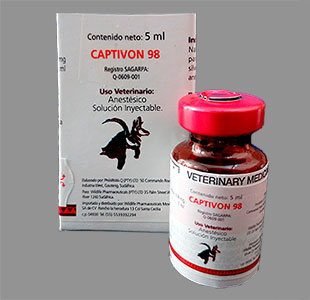 |
Immobilon© (Etorphine) is sometimes supplied with the antidote Revivon© (Diprenorphine). Another trade name for etorphine is Captivon 98©
Data on etorphine toxicity are conflicting, according to one report (Riviere J. E., 2013) the lethal dose for humans is only 0.03–0.12 mg.[7] According to others (Jasinski D. R. et al. 1975) — 0.1 mg of etorphine administered subcutaneously causes only marked euphoria and pupil constriction.[8]
In the homeland of etorphine, in the UK, the military potential of "Bentley compounds" was studied from their discovery until the early 1970s. The research was carried out at the Chemical Defense Experimental Establishment (CDEE) in Porton Down. As it turned out, some thebaine derivatives caused severe dizziness in humans and CDEE considered them as potential incapacitants. One of these compounds, codenamed TL 2833, had an immediate but brief "knockout" effect in addition to dizziness. Its trials were conducted in 1963–1965 and were terminated due to the pronounced depressing effect of the drug on the cardiovascular system — the introduction of TL 2833 in doses over 0.1 mg caused a decrease in blood pressure, and one of the volunteers had symptoms of catatonia.[17]
In 1993, know-how technology for the industrial synthesis of etorphine was purchased for $150 000[5] for laboratories developing chemical weapons in the Republic of South Africa.
In 2002, during a hostage rescue operation, an unknown narcotic gas was used in the Dubrovka Theater Center. When no traces of fentanyl derivatives were found in the blood and urine tests of the two German hostages[9], therefore some Western toxicologists suggested that the active substance of the Russian "knockout gas" could be etorphine.[21] As it turned out later, however, the "knockout gas" included the opioid analgesics carfentanil and remifentanil.
In the Czech Republic in 2005, the possibility of using a combination of etorphine and the opioid agonist-antagonist butorphanol as a "psychopharmacological weapon" was studied, and experiments were conducted on transdermal administration of etorphine using dimethyl sulfoxide (DMSO). Experiments by Czech scientists have shown that solutions of etorphine in DMSO, when applied to the skin, cause immobilization in as little as 3–8 minutes.[6]
The incapacitants of the thebaine group cannot be used in aerosol form because of their high toxicity, but etorphine or its derivatives were probably used in the Chinese non-lethal BBQ-901 system for firing "tranquilizer darts." This is a specialized weapon for use by intelligence units for covert capture and by public security forces for the immobilization of criminals. BBQ-901 is a gun for firing "flying syringes" with anesthetic at a distance of up to 40 meters.
|
Anesthesia system model BBQ-901
 |
"Flying syringes" for BBQ-901 system
 |
Anaesthetic system model BBQ-901 manufactured by Chinese company NORINCO and its cartridges
BBQ-901 has been developed in China since the 1980s, and in those years etorphine had no competitors in terms of speed of onset of effect. According to the advertising prospectus, when the domestically produced (Chinese) drug is used in the dart, the effect occurs within tens of seconds. After immobilization, administration of an antidote is required, after which the subject regains full consciousness in 2–3 minutes.[23] The BBQ-911 system was last seen at a gun show in Hong Kong in 2014. The composition of the anesthetic is undisclosed, but newer modifications have likely started using safer derivatives of fentanyl.
RX 140M. An isoamyl homologue of etorphine, was studied in the early 1970s in the United States and was considered a more suitable candidate for "sleeping gas" than etorphine. RX 140M is superior to etorphine in potency of narcotic action and has a more favorable pharmacological profile.[13]
In 1976, the Swiss Armament Technology and Procurement Group investigated the effect of thebaine derivatives on respiratory function. A total of 31 compounds were studied, of which eight were 4–10 times more potent than etorphine in terms of analgesic activity. Among these most potent analgesics, RX 140M had the highest therapeutic index (LD50/ED50).[3]
Dihydroetorphine (DHE) is 3-6 times more potent analgesic than etorphine and with higher selectivity towards μ-receptors.[10] Experiments conducted by Chinese scientists in 1982 showed that DHE at a dose of 0.0001–0.0005 mg/kg caused analgesia in monkeys, dogs, rabbits, and mice. DHE, like most opioids, has an incredibly high therapeutic index (LD50/ED50) in rodents — 115 000, and low in primates — the lethal dose for monkeys is only 50 times the analgesic dose.[10] The DHE research was conducted at the Beijing Institute of Pharmacology and Toxicology of Academy of Military Nedical Sciences.[18]
After it was found that physical dependence in animals develops only when high doses of DHE[11] are administered, it began to be used in China in 1992 for the treatment of opiate dependence. DHE was available in the form of sublingual tablets of 0.02–0.04 mg to be taken every 3–4 hours.[12]
Thienorphine. After the introduction of dihydroethorphine into medical practice, Chinese scientists continued research on thebaine derivatives, but of their own design. The first successful drug was Thienorphine (Thienorphine), which was intended as a treatment for opioid addiction. The new compound had a unique pharmacological profile — antagonism to the effects of morphine persisted for at least a week after a single injection. In 2020, thienorphine was in phase II clinical trials.[19]
Thienorphine proved to be a fairly weak analgesic, but the next Chinese drug TH-030418 is a potent opioid, 5 000 times more potent than morphine and effective for about 12 hours.[20]
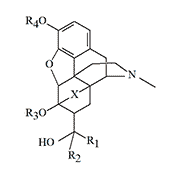 |
||||||
| X | R1 | R2 | R3 | R4 | Analgesic Activity (Morphine=1) |
Name or Designation |
|---|---|---|---|---|---|---|
| CH=CH | H | Ph-CH2CH2- | Me | H | 1100 | |
| Me | i-Am | Me | Ac | 1300 | ||
| Me | Cyclohexyl | Me | Ac | 1700 | ||
| Me | Ph-CH2CH2- | Me | H | 2200 | ||
| Me | n-Pr | Me | H | 3200 | Etorphine (M-99) | |
| Me | Cyclohexyl | Me | H | 3400 | TL 2636 (M-125) | |
| Me | n-Am | Me | H | 4500 | ||
| Me | n-Bu | Me | H | 5200 | ||
| Me | n-Pr | Me | Ac | 8700 | Acetorphine (M-183) | |
| Me | i-Am | Me | H | 9200 | RX 140M (M-140) | |
| CH2 —CH2 | Me | 3-thienyl-CH2 CH2- | Me | H | 5000 | TH-030418 |
| Me | i-Am | Me | H | 11000 | ||
| Me | n-Pr | Me | H | 12000 | Dihydroetorphine | |
According to K. W. Bentley, D. G. Hardy (1967)[15].
Among the derivatives of thebaine there are psychotomimetics, not inferior in hallucinogenic action to LSD, there are substances causing vestibular disorders and even emetics - the thebaine derivative M320 is four times more active than apomorphine in its vomiting action.[22]