Methaqualone and its Derivatives
 |
 |
Wouter Basson (pictured on the right) led the South African Chemical and Biological Warfare program in the 1980s and 1990s. Narcotic substances such as Methaqualone, Ecstasy, Tetrahydrocannabinol and Angel Dust (PCP) were considered as potential incapacitating agents. In 1998, he appeared before the Truth and Reconciliation Commission but was acquitted in 2002. (On the left is a caricature SAHA)
Methaqualone: A Failed Medicine and a Dangerous Drug
Methaqualone is a sedative-hypnotic medication from the quinazolin-4(3H)-ones group. It belongs to the class of non-selective positive allosteric modulators at GABAA receptors.[23] First synthesized by Indian chemists in 1951[1], but its hypnotic effect was only discovered in 1955.[2] As a sleeping aid, methaqualone was considered safer than the barbiturates used at the time, as it was less likely to cause respiratory depression and hypotension in cases of overdose.[3] The therapeutic dose for sleep induction is 300 mg, while the recreational dose ranges between 150–300 mg. The lethal dose of methaqualone is around 8000 mg.[5]
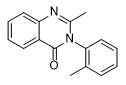
Methaqualone
2-Methyl-3-(2-methylphenyl)-4(3H)-quinazolinone
If individuals resisted the urge to sleep, methaqualone induced sedation, relaxation, euphoria, and notable increases in sexual arousal, which made it a “love drug.” In the 1970s, methaqualone rapidly gained popularity as a recreational drug among the youth in Germany, Japan, and, slightly later, the United States. Tolerance to methaqualone developed quickly, and prolonged use of high doses led to physical dependence. It was often combined with alcohol, significantly enhancing methaqualone’s toxicity. In the United States, in 1980, 117 people died after consuming illegally purchased methaqualone.[4] Pharmaceutical production of methaqualone ceased in 1983, and the following year it was classified as a Schedule I drug, prohibiting any medical use.
 |
 |
Methaqualone was sold in the United States under the brand name Quaalude (a wordplay on 'quiet interlude'). An important advantage of Methaqualone (Quaalude) over barbiturates and other sleeping aids was the absence of a hangover the following morning after use
The boom in online trade of new psychoactive substances that began in the early 2010s did not revive the popularity of methaqualone or its few quinazolinones. Seizures, loss of motor control, amnesia, and sudden loss of consciousness during smoking — these side effects from quinazolinones led to negative reviews on forums and a decline in interest in this group of designer drugs.[6] Today, methaqualone is primarily found in South Africa, where it is often smoked in combination with cannabis.
Synthesis. The synthesis of methaqualone does not require rare or expensive precursors — the main components are anthranilic acid and o-toluidine. Theoretically, it could also be synthesized from toluene, sulfuric, nitric, and acetic acids.[32] However, such production would only be cost-effective at scales of tens or hundreds of kilograms. In India, clandestine production of methaqualone has reached an industrial scale, in 2016, one of the largest narcotic drug seizures in the world took place there, with 23,500 kg of Mandrax tablets (methaqualone) confiscated.[7]
Toxicology. Methaqualone have a relatively narrow therapeutic index, which often leads to overdoses. However, the most common cause of death was not overdose but rather fatal trauma (72%). One-third of victims died in vehicular crashes.[8] These accidents were attributed to a "delusion of sobriety" — a false belief that one is completely sober despite clear evidence to the contrary, such as severe cognitive impairment and significant loss of motor control.
In cases of severe methaqualone poisoning, the most common symptoms are loss of consciousness and seizures. The severity of consciousness impairment depends on the dose. In one case, a coma lasted 3 hours after ingesting 4 grams, while in another, the patient regained consciousness nearly four days after taking 7.5 grams.[5]
Convulsive readiness is one of the main signs of methaqualone overdose, distinguishing it from other hypnotic and sedative drugs. Its intensity ranges from tremors, muscle twitching, or muscle spasms to frank convulsions[5,9].
In cases of methaqualone poisoning, there may be a reduction in blood clotting, leading to retinal hemorrhages, purpura, and gastrointestinal hemorrhages[12].
SHORT-TERM BEHAVIORAL EFFECTS OF METHAQUALONE
| Low Dose (75 mg) |
Moderate Dose (150 mg-300 mg) |
High Dose (over 300 mg) |
|---|---|---|
| Calmness Relaxation Dizziness Tingling Numbness of extremities Drowsiness Restlessness Anxiety Burning |
Euphoria Increased sociability Tingling or numbness throughout the body Self confidence Sexual arousal |
General numbness Weakness Fear of “losing one’s mind” Incoordination Agitation Panic |
PHYSIOLOGICAL EFFECTS OF METHAQUALONE IN SHORT-TERM, NONTOLERANT USERS
| Low Dose (75 mg) |
Moderate Dose (150 mg-300 mg) |
High Dose (over 300 mg) |
|---|---|---|
| Drowsiness; sleep Fatigue Restlessness Headache Perspiration Dry mouth Loss of appetite Nausea; vomiting Stomach discomfort Diarrhea |
More intense low-dose effects | Sleep Tremors Muscle spasms Profuse perspiration Rapid heart rate Motor incoordination Slurred speech Amnesia Chills Respiratory depression |
Source: Addiction Research Foundation, Canada[31]
Therapy. The primary treatment for methaqualone poisoning is intensive supportive therapy.[9] Due to the high lipophilicity of methaqualone, it quickly accumulates in fatty tissues, making it difficult to remove from the bloodstream through detoxification methods such as hemodialysis and hemoperfusion[5]. The use of forced diuresis is also risky due to the potential for pulmonary edema.[9] In methaqualone derivative poisoning, the seizure syndrome may sometimes be accompanied by psychomotor agitation, which can be treated with benzodiazepines. In cases of severe seizures, neuromuscular-blocking drugs and mechanical ventilation may be required.[5]
To stop bleeding, complex treatment may be required, including platelet transfusion, fresh frozen plasma, and vitamin K administration.[12]
Methaqualone as an Incapacitant: Make love, not war
Research into methaqualone as an incapacitant began in South Africa as part of the secret offensive CBW program known as Project Coast in the early 1980s. In 1983, the South African Police handed over 20,000 Mandrax tablets to the military. In classified documents, methaqualone appeared under various codenames — Product M, Mx, FP/00MO1, or MosRefCat (Mossgas Refinery Catalyst). The plan was to use this new non-lethal chemical agent to arm 81-mm mortar shells (containing 500 grams of methaqualone), hand grenades (350 grams of methaqualone), and 155-mm artillery shells.[13]
The "love drugs" methaqualone and MDMA (Ecstasy) developed under Project Coast as incapacitating agents were not intended to quickly knock out rioters but rather to reduce their aggression and induce calm.
"The philosophy … was that under certain circumstances one could provide or use sedatives which could possibly decrease the anger of the crowds."
"If I could give Ecstasy to every person, he will not make war, but love, then I can in a matter of 10 minutes, change his whole spiritual condition."
Erstwhile SA Police forensics chief, Lieutenant-General Lothar Neethling[14]
In 1986–87, methaqualone was tested on volunteers, who were exposed to the smoke while engaged in a simulated battle:[14]
However, overall, the results of the experiments on humans and animals were "disappointing," and in 1988, the production of methaqualone was discontinued. As a result of these tests, “a far more active analogue" was sought, to overcome certain drawbacks.[13]
According to project head Wouter Basson, the methaqualone analogue developed by Project Coast, tested on SA Police until 1988, stood out due to its effect of heightened aggression that persisted for days after exposure. An enhanced version of methaqualone was reportedly tested in 1993. Physiological experiments were conducted on animals, while human trials involved subjects placed in an enclosed room where smoke was released.[15]
In 1992, the Coordinating Management Committee of Project Coast decided to procure methaqualone from abroad. Wouter Basson, using his connections, attempted to purchase a batch of methaqualone from the USSR. However, it was discovered that the Russian Academy of Sciences could not fulfill the order and recommended contacting their colleagues in Croatia. In 1992, Basson purchased in Croatia 600 kg of a substance labeled as "Quinezoolione" in the documents, at a price of $5,000 per kilogram.[13] It remains unknown whether "Quinezoolione" was the original methaqualone or an improved version.
The use of methaqualone as an incapacitating agent seems like a strange and reckless adventure. Indeed, methaqualone vaporizes well upon heating and could be used in pyrotechnic formulations, but that is perhaps its only useful property. As an incapacitant, it is almost 1000 times less effective than psychochemical Agent BZ, which is also used in smoke form. Additionally, methaqualone proved to be too addictive and toxic, leading to its gradual replacement by safer benzodiazepines. Animal studies have shown that the lethal dose of methaqualone is only five times the hypnotic dose[16], and for humans, the safety margin is only 14 or 15, while for a dependent user, it is closer to 2 or 3.[17] Chemical agents with such toxicity levels cannot be considered non-lethal chemical weapons.
During the trial of Wouter Basson, several alternative theories were discussed regarding his heightened interest in methaqualone and MDMA. According to one version, methaqualone was intended for illegal sale, with the proceeds potentially funding secret operations or contributing to Wouter Basson's personal enrichment. Another version suggested that methaqualone was the most popular drug among the Black population of the Western Cape Province, and the mass influx of large, cheap quantities of methaqualone into black markets would have led to increased drug addiction and reduced protest activity among African youth[14]. However, despite numerous charges, the court was unable to prove Wouter Basson's guilt.
 |
 |
South African users would smoke the mixture of weed and methaqualone through the neck of a broken glass bottle. This combination is known as "white pipe." One of the Project Coast initiatives focused on studying the combined effect of methaqualone and cannabis for potential use as crowd control agents.[13]
It is unclear which "far more active analogue" of methaqualone was ultimately chosen by the researchers of Project Coast. At the time, the most potent commercially available quinazolinone was Nitromethaqualone. The hypnotic dose of this substance is 6–10 times lower than that of methaqualone,[18] and when smoked or vaporized, the effects are felt after inhaling just 5 mg of nitromethaqualone.[6] Despite the lower dose, as a sleeping aid, nitromethaqualone was less effective than methaqualone.[18] Among drug users, nitromethaqualone raised serious concerns due to the suspected mutagenic effects[19] of its metabolite 2-methoxy-4-nitroaniline.[20]
The dosages of quinazolinones that cause sedation and euphoria when inhaled (smoked or vaporized)
 |
 |
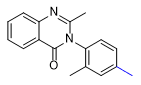 |
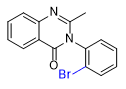 |
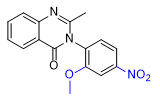 |
| Etaqualone 50–80 mg[21] |
Ephinazone 35–50 mg[21] |
Methylmethaqualone 40–55 mg[21] |
Mebroqualone 15–30 mg[21] |
Nitromethaqualone 5 mg[6] |
Methaqualone, MDMA, and tetrahydrocannabinol were produced on an industrial scale for the needs of South Africa's chemical program. The idea of using euphoriants and empathogens to suppress mass unrest was deemed highly questionable by the judges, but the accusing party was also unable to provide convincing counterarguments.
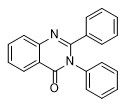
PPQ and New Ultra-Potent Quinazolinones
Quinazolinone derivatives were unsuitable for temporarily incapacitating due to two main drawbacks: high toxicity and a high incapacitating dose. However, one of the drawbacks of quinazolinone incapacitating agents — low activity — seems to have been overcome. In 2018 and 2020, researchers from the University of Copenhagen published two articles on the exceptionally active substituted 2,3-Diphenylquinazolin-4(3H)-one (PPQ).[22] PPQ and its 3-(p-tolyl)-analog (PPTQ)[23] displayed 40−160-fold increases in modulatory potency compared to methaqualone across α1-5β2γ2S GABAA-receptors.[22]
 |
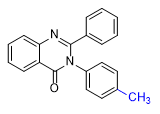 |
| PPQ Diphenylquinazolin-4(3H)-one 5–10 mg[24c] |
PPTQ 2-Phenyl-3-(p-tolyl)quinazolin-4(3H)-one 2–7 mg[24e] |
As it soon became clear, the high modulatory potency of PPQ analogs in vivo correlates with powerful sedative-hypnotic effects in humans. In 2023, a talented amateur chemist synthesized PPTQ and tested its effects on himself[24e]. PPTQ produced the same effects Wouter Basson anticipated from methaqualone: sedation, opioid-like euphoria, muscle relaxation, and impaired coordination, but at a dose 100 times smaller than methaqualone[24e]. In 2024, the same anonymous chemist synthesized and tested on himself at least a dozen and a half PPQ derivatives[24a-d].
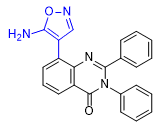
According to him, 8-substituted PPQs in the quinazoline core have a reduced sedative effect compared to methaqualone but exhibit a similar aphrodisiac effect[24a]. One of the substances in this series is a hybrid of the molecules of methaqualone and muscimol, a psychoactive compound from fly agaric (Amanita muscaria).
 |
 |
 |
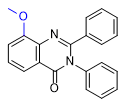 |
| 4–8 mg[24a] | 3–6 mg[24a] | 3–6 mg[24a] | 2–4 mg[24a] |
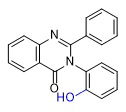
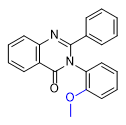
2-Cl- and 2-Me-PPQ have effects similar to methaqualone, with a dose of 1–1.5 mg of these substances being equivalent to 300 mg of methaqualone[24A].
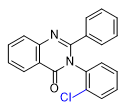 |
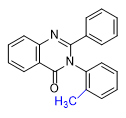 |
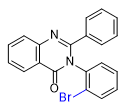 |
 |
 |
| 0.5–1.5 mg[24a] | 0.5–1.5 mg[24a] | 0.75–1.5 mg[24c] | 4–8 mg[24c] | 1.25 mg[24c] |
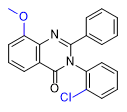
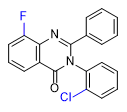
Substitution at the 8-position of the quinazoline core combined with a halogen in the ortho position of the 3-phenyl group further enhances the activity of PPQ analogs. These compounds exhibit reduced sedative effects but more pronounced euphoria[24D].
 |
 |
 |
 |
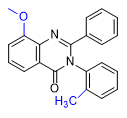 |
| 1–2 mg[24a] | 0.4–0.8 mg[24a] | 0.2–0.5 mg[24a] | 0.3–0.6 mg[24a] | Mephenaqualone 2–4 mg |
Mephenqualone (Mephenqolon) emerged on the German gray markets in mid-2024. This substance is extremely dangerous due to its unpredictable effects. Individual sensitivity to it can vary significantly: some subjects experience euphoria and sedation after ingesting as little as 0.7 mg, while others show no reaction even after use of 40 mg.[33]
Like methaqualone, PPQ analogs are easily sublimated. Inhalation doses of PPQ analogs are generally about 50% of the oral doses.[24]
Compounds containing the quinazolin-4(3H)-one structure exhibit a wide range of pharmacological activities and have been actively studied in recent years. However, derivatives of PPQ are rare among them — only a few compounds with anticancer and anticonvulsant properties have been described,[25,26] with no mention of any sedative-hypnotic effects.
In 2021, Iran, the People's Republic of China, Russia, and Syria objected to the decision adopted by the OPCW Conference regarding the aerosol use of chemicals affecting the central nervous system (CNS).[27,28] In two of these countries, Iran and China, active research is being conducted on highly potent PPQ analogs.
IRAN According to the Office of the Director of National Intelligence (ODNI) report published in 2024, Iranian military scientists have investigated chemicals, toxins, and bioregulators exhibiting diverse pharmacological properties, including sedative, dissociative, and amnestic incapacitating effects.[34] Since the early 2000s, Iranian chemists have shown increasing interest in various methods for synthesizing quinazolin-4(3H)-one derivatives, including one-pot reactions, microwave irradiation, solvent-free conditions and others.[10]
The synthesis of 4(3H)-quinazolinones was part of "continuing efforts on the development of new routes for the preparation of biologically active heterocyclic compounds".[10m] However, it is surprising that Iranian scientists have never published data on the exceptionally high biological activity of these compounds, although PPQ was synthesized in 1998[10a] and PPTQ, 2-Me-PPQ, 2-Cl-PPQ and analogs in 2004[10c-d].
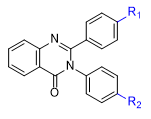 |
R1 | R2 |
|---|---|---|
| –H | –H, –Me, –OMe, –Cl | |
| –Me | –H, –Me | |
| –Cl | –H, –OMe, –Cl | |
| –NO2 | –Me |
Derivatives of methaqualone synthesized in the Iranian project on the “Preparation of Biologically Active Heterocyclic Compounds” (2012)[10k]
Additionally, it is concerning that researchers from the Chemistry and Chemical Engineering Research Center of Iran (CCERCI) are involved in these studies. According to Iranian opposition sources, CCERCI is linked to Iran’s program for the development of non-lethal chemical agents[29], such as fentanyls, adrenomimetics, and irritants. Chinese experts are reportedly overseeing the research.
People's Republic of China (PRC) Various PPQ derivatives were synthesized at the Academy of Military Medical Sciences (AMMS) between 2017 and 2020.[11c-g] In 2017, one of the AMMS researchers accidentally discovered the good sedative and hypnotic effects of the 2-(m-fluorophenyl) analog of PPQ (ZXW 7046).[11c] The substance was several times more potent than methaqualone, but at hypnotic doses, it caused seizures in mice. Chinese chemists synthesized dozens of PPQ analogs with different halogen and methyl groups, both in the phenyl rings and in the quinazolinone core. All of them demonstrated strong hypnotic properties, but most (including PPQ and Me-PPQ) caused seizures followed by paralysis in animal subjects. In 2019, AMMS chemists achieved success by making two important conclusions about the relationship between structure and the hypnotic activity of 2-phenyl-substituted PPQs:
- atom Cl in the ortho position is crucial for improving drug efficacy;[11d,e]
- the methyl group in the para position prevents of seizures and paralysis. Due to rapid metabolism into a methoxy group, the synthesized substances have a short duration of action — 25 minutes.[11g]
Adding fluorine to the quinazolinone core (ZXW 1646) or replacing the 2-phenyl group with a cyclopentyl or cyclohexyl group (ZXW 1624) did not reduce activity. These substances exerted hypnotic effects at doses 10 times lower than methaqualone in animal.[11g] For humans, the effective doses of these compounds are likely similar to those of PPQ and PPTQ, in the range of 1–10 mg.
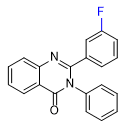 |
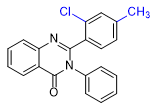 |
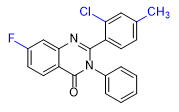 |
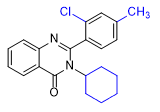 |
| ZXW 7046 | ZXW 9050 | ZXW 1646 | ZXW 1624 |
Addition of a halogen or nitro group to the 2-phenyl ring, as in compounds synthesized by Danish researchers, may further increase activity.
Summary
Countries such as Iran, India, and the People’s Republic of China are likely still considering lethal poisons like fentanyls as incapacitating agents. However, methaqualone analogs are even worse candidates for non-lethal chemical agents. Victims of fentanyl overdoses can be saved if an antidote (naloxone) is administered in time. In cases of methaqualone and its analog overdose, there is no antidote.
Despite the lack of prospects for the use of new methaqualone analogs as incapacitating agents, there is a potential threat of their use for narco-terrorism by countries such as the People's Republic of China. One only needs to recall the time and effort the United States expended to curb the smuggling of fentanyl from China. Ultimately, Chinese companies shifted to supplying precursors for its production to Mexican cartels.
In the past, the Eastern Bloc has already used methaqualone in its struggle against capitalist adversaries. The methaqualone tablets that flooded the U.S. in the early 1980s were manufactured in Colombia from powder supplied by China and Hungary.[30] Czechoslovakia was a another significant producer of methaqualone and a supplier to the black market. However, under pressure from the United States, the country halted production in 1982.[31] Another socialist country, Cuba, was involved in the methaqualone trafficking route from Colombia to the U.S.[30]