Urticants II. Nettle Chemical Agents
In addition to phosgene oxime, other oximes were studied as Nettle Gases, which caused instant burning pain upon contact with skin and mucous membranes. German intelligence believed that this group of CW agents was being actively studied in the USSR before the World War II.[94] However, for various reasons, none of them were ever adopted for use.
Halogenated Oximes Possessing Urticant Effects
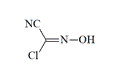
Monochloroformoxime (CY, formyl chloride oxime) appears as a crystalline substance with a pungent odor reminiscent of hydrogen cyanide. It demonstrates extreme chemical instability, undergoing explosive decomposition at 40ºC. Vapor exposure induces inflammation of ocular mucous membranes, salivation, pharyngeal irritation, and severe cephalgia. Cutaneous exposure results in the formation of white vesicles and deep, slowly-healing lesions. The compound's extreme instability at ambient temperature precludes its practical application as a CW agent.[66]
Chlorocyanoformoxime was first synthesized by W. Steinkopf in 1911, who documented its irritant properties. Chlorocyanoformoxime induces lacrimation and exhibits potent corrosive effects on mucous membranes, resulting in vesication.[37] Chlorocyanoformoxime was thoroughly investigated but was not adopted by the Soviet chemists probably because of its feeble stability, despite its good itching action.[94]
Dibromoformoxime (CV) The compound is synthesized via the reaction of formamide with bromic acid in aqueous or alcoholic solution. Dibromo- and Diiodoformoxime were found as effective as Phosgene Oxime.[94]
 |
 |
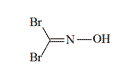 |
|
| Monochloroformoxime (CY) | Cyanochlorformoxime | Dibromoformoxime (CV) |
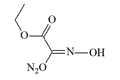
Ethyl Isonitrosoacetate. This compound was first synthesized in 1895 by German chemist M. Jovitschitsch, who documented its "corrosive effects on skin" and noted that "during handling, it causes severe ocular irritation".[89]
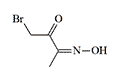
Diacetyl Monoxime Bromide. First synthesized by Diels & Farkaš in 1910. Chemists working with the compound observed that "in dissolved state, this compound is easily volatile with the vapors of the solvent and strongly attacks the eyes and mucous membranes. On the skin, it causes purulent inflammations".[88]
Chloroisonitrosoacetone, a precursor to phosgene oxime, was first synthesized in 1925.[90] The compound underwent evaluation in the United States as a potential vesicant agent in 1926. It induces severe dermatological lesions progressing to tissue necrosis. In 1939, Italian toxicologist M. Milone, in his publication "On the Urticant Effects of Chloroximes," postulated the potential military application of chloroisonitrosoacetone as a CW agent.[68]
SKIN BLISTERING EFFECT OF AQUEOUS SOLUTIONS OF CHLOROISONITROSOACETONE.[68]
| 1–2% | Slight reddening |
| 3% | Reddening lasting longer, a light inflamation of the skin within from minutes, often tiny blisters appearing |
| 15% | A drop cause immediate, severe painful irritation. Blisters appear, followed by edema. Healing after 40 days leaving pigmented sears |
| 40–50% | Concentrations cause often some necrosis |
As a CW agent, chloroisonitrosoacetone was studied in Germany in 1938–1939. However, despite its strong nettle-like effect, the inhalation of 500 mg/m3 per 30 minutes had no physiological effect, it caused only a slight irritation.[94] Dichlorodinitrosoacetone exhibits similar biological effects and additionally induces cutaneous depigmentation.[26]
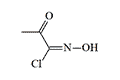
Sulphuryl Chloride Phosgene Oxime was synthesized at the Military Academy of Chemical Defense (Kostroma, USSR) in 1965. The compound exhibits lachrymatory properties with relatively moderate toxicity.[52] German literature references this substance as a binary formulation of phosgene oxime and chlorosulfonic acid, with potential applications as a toxic smoke agent.[49] Notably, sulphuryl chloride serves as a decontaminating agent for persistent chemical warfare agents.
 |
 |
 |
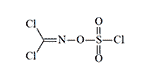 |
| Ethyl Isonitrosoacetate | Diacetylmonoxime bromide |
Chlorisonitrosoacetone | Sulfurylchloride Dichloroformoxime |
Trichloromethyl chloroformoxime synthesized by Nazi military chemist H. Brintzinger in the in the 1940s was characterized by "identical physiological properties (stinging effect, blister formation) to phosgene oxime, and represents a completely stable compound capable of long-term storage without decomposition".[65]
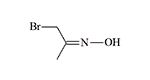
Bromoacetone oxime |
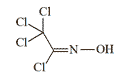
Trichlormethylchlorformoxime |
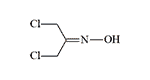
Bis(chlormethyl)formoxime |
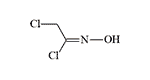
Chlormethylchlorformoxime |
Two additional compounds in this group, bis(chloromethyl)formoxime and Chlormethylchlorformoxime, exhibit urticarial effects and demonstrate greater stability compared to phosgene oxime, though they ultimately degrade over time.[65] Comprehensive toxicological data for this class of urticants remains unavailable.
Trichlormethylchlorformoxime, chlormethylchlorformoxime, and cyanformylchloroformoxime have been attempted to be used to stabilize phosgene oxime, but the addition of substances having a more feeble action on the skin has diminished considerably the effectiveness of phosgene oxime.[94]
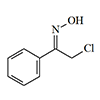
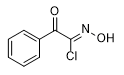
Chloro- and Bromoacetophenone Oximes were first synthesized by German chemists H. Korten & R. Scholl in 1901, who personally documented their severe physiological effects. Cutaneous exposure to crystalline forms or solutions of these oximes induced sustained burning sensations, while vapor exposure resulted in severe ocular irritation.[39] According to K. Los research, chloroacetophenone oxime demonstrates potent lachrymatory properties and induces severe, persistent dermatological lesions.[40] M. Milone analysis suggests these compounds cannot be classified as true "nettle agents",[68] because their burning effect on the skin is relatively mild. [66] Chlorisonitrosoacetophenone has the same weak irritant effect. [66]
 |
 |
 |
| Chloracetophenone-oxime | Chlorisonitroso acetophenone |
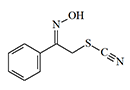
Acetophenone Thiocyanide Oxime |
Acetophenone Thiocyanide Oxime similarly exhibits dermal irritant properties.[41]
Other Nettle compounds
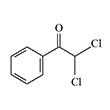
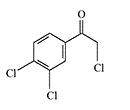
Chlorinated acetophenone derivatives, such as α,α-dichloroacetophenone, ω,3,4-trichloroacetophenone, and ω-trichloroacetophenone, exhibit strong vesicant properties and have been repeatedly considered as potential chemical warfare agents.
 |
 |
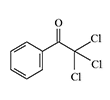 |
| α,α-Dichloroacetophenone | ω,3,4-Trichloroacetophenone | ω-Trichloroacetophenone |
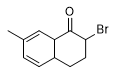
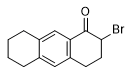
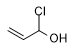
In the literature on military chemistry, 2-brom-1-oxo-7-methyltetrahydronaphthalene, 2-brom-1-oxo-octahydroanthracene and α-chloroallyl alcohol, are mentioned, which cause severe skin burning.[94]
 |
 |
 |
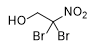 |
|
| 2-Brom-1-oxo-7-methyl tetrahydronaphthalen |
2-Brom-1-oxo- octahydroanthracene |
α-Chloroallyl alcohol |
2,2-dibromo-2-nitroethanol | Dichloroacetone |
Other substances known to induce urticant effects include 2,2-dibromo-2-nitroethanol[94] and dichloroacetone.[66] The irritant and blistering effects of dichloroacetone were studied by military chemists in the USSR and the USA during the 1920s and 1930s.(see details).
Aromatic Organoarsenic Compounds
Unlike vesicant agents like lewisite, methyl-, ethyl- and phenyl- dichloroarsines which have delayed effects and mild irritant properties, there exists a group of aromatic arsines that cause immediate pain response upon contact with skin and mucous membranes, combined with severe upper respiratory tract irritation.
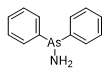
The Soviet chemical defense manual (1942) lists phenylarsinimine and arsacridine chloride as nettle agents.[38] Phenylarsinimine and diphenylarsinamine, which also has irritant effects on skin and mucous membranes, were described by the notable Russian chemist W. Ipatiew in 1928.[96] From 1916, Professor W. Ipatiew led research on CW agents first in the Russian Empire and later in the Soviet Union.
 |
 |
||
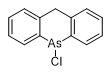
| Phenylarsinimine | Diphenylarsinamine | Excelsior |
Excelsior (Chloro-5,1-dihydroacridarsine, EX, Arsacridine) was synthesized in 1930. In addition to its irritant effects on the upper respiratory tract, it "when dusted in the air in smallest amounts, causes severe burning of the face, the lips and the tongue".[95]
Today, "nettle gases" have completely lost their military significance; as a non-lethal alternative, they have been replaced by irritants CR and CH, which cause an intense pain reaction but do not cause skin damage (blisters, necrosis).