Incapacitants of the Fentanyl Group

This is what 2 milligrams of fentanyl looks like — a lethal dose for most people.
In 1960, Belgian pharmacologist Paul Janssen synthesized a new extremely potent analgesic, which later became known as Fentanil.[40] In animal experiments, fentanyl induced analgesia at doses hundreds of times smaller than morphine, for humans, fentanyl is 50–100 times more effective than morphine.[35] After intravenous administration of 0.05–0.1 mg of fentanyl, analgesic effects occur within 1–2 minutes and last for 30–60 minutes. At doses above 0.2 mg, fentanyl causes anesthesia and respiratory depression.
UNITED STATES In the early 1960s, the Chemical Warfare Center at Edgewood Arsenal began researching fentanyl and other potent morphine-like substances. The first trials of fentanyl on volunteers, in which the yet-to-be-patented fentanyl was designated with the internal code CS 42251, were conducted in 1963.[5] For medical use in the United States, fentanyl was approved only five years later, in 1968.
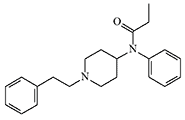 |
 |
Fentanyl. Chemical formula and dosage forms
During the Vietnam War, in the second half of the 1960s, the US Military Advisory Command Studies and Observation Group (MACV-SOG) considered using fentanyl for tactical roles in Vietnam. SOG’s mission to capture enemy officers for interrogation was challenging, especially along the Ho Chi Minh trail, where supply carriers were often low-ranked soldiers or peasants, but high-ranking North Vietnamese Army officers were also present. Attempts to capture these officers often resulted in firefights and casualties among civilians. Commanders of SOG John Singlaub proposed using tranquilizing darts with fentanyl to temporarily incapacitate targets while dispersing the rest. However, General Westmoreland’s science advisor did not approve this plan, and only the riot control agent CS was authorized for use in the region.[20]
Potential Military Chemical/Biological Agents and Compounds. Army Field Manual No 3-9 (1990) describes the effects of fentanyls on humans as follows:
The U.S. Department of Defense showed interest in fentanyl analogs at least until the mid-90s,[6,30] but gradually they were replaced by what were considered more effective and safer 4-substituted derivatives, such as carfentanil and remifentanil.
SOVIET UNION. Research on the potential use of fentanyls as incapacitating agents was also conducted in the USSR. According to Major General N.S. Antonov, who headed the military-chemical research complex in Shikhany for many years, fentanyls were considered the most promising class of non-lethal chemical weapons.
In 1963, the KGB approached the Central Committee of the Communist Party and the Council of Ministers with the initiative to organize the production of psychoactive chemical agents in the USSR. Pilot production of incapacitating agents was handled by one of the branches of the State Scientific Research Institute of Organic Chemistry and Technology GosNIIOKhT near Saratov [65] and The Institute of Organic Synthesis, Academy of Sciences, Latvian SSR, was one of six research centers developing new non-lethal chemical agents.[65]
In the 1960s, the Chief Anesthesiologist of the Latvian SSR, Dr. Georgs Andrejevs, approached the Institute of Organic Synthesis with a request to develop an industrial process for manufacturing fentanyl. He explained this was due to a shortage of the drug caused by limited supplies from Russia.[62] It was a very strange proposal since Latvia would not have been able to start commercial production anyway, due to all rights to produce fentanyl being held by the American company Johnson & Johnson until 1981. It is possible that the industrial method for obtaining this powerful analgesic was needed for other purposes as well — at that time, Georgs Andrejevs was an informant and scientific consultant for the Committee for State Security (KGB).[67]
At the Latvian Institute of Organic Synthesis, a unique and incredibly inexpensive method for producing fentanyl was developed — one kilogram of fentanyl produced in the laboratory cost around $3000. In industrial production, the cost would be even lower. No pharmaceutical company in the USSR was willing to produce cheap fentanyl at $0.0015 per ampoule — it was simply too unprofitable. The production of Soviet fentanyl began only after fraudulent manipulations increased the cost of 1 kilogram of the substance by 50 times(!), to $150,000.[62]
Fentanyl powder from Riga was sent to the Latvian plant "Sanitas," where it was converted into citrate and the solution was filled into ampoules, up to 10 million ampoules per year. The secret laboratories of "Sanitas" fulfilled special orders for the 13th Department of the KGB, whose responsibilities included the elimination of enemies of Soviet power abroad.[66] The Latvian method also allowed for the production of other, more potent fentanyl analogs,[62] which could be used as chemical weapons or sabotage poison. In 1988, a fentanyl derivative, believed to be of Soviet origin by Western intelligence agencies, was likely used to assassinate President of Pakistan Mohammed Zia ul-Haq, who had supported the Afghan mujahideen (See details).
Cases of the Soviet army using narcotic gases, likely based on fentanyls, were documented during combat operations in Laos, Cambodia, and Afghanistan. The definitive confirmation of the presence of fentanyls in Russian chemical arsenals came with the use of a carfentanil and remifentanil aerosol during the storming of the Dubrovka Theater in Moscow in 2002 (See details).
After the dissolution of the USSR, the production of fentanyl was moved from the Baltic countries to Russia. Currently, the main producer of pharmaceutical-grade fentanyl is the State Scientific Research Institute of Organic Chemistry and Technology (GosNIIOKhT),[69] and it is likely that they also produce fentanyl derivatives for the Russian military and special services. Since 2000, GosNIIOKhT has been under US sanctions for developing and testing chemical weapons.
Phenaridine (2,5-dimethylfentanyl) was an attempt in the USSR to introduce a highly potent opioid analgesic into medical practice between 1991 and 1996. Its industrial production could have been easily established since it shared a common precursor with Promedol, but Phenaridine was several hundred times more potent.[60] However, Phenaridine exhibited more severe side effects and did not pass clinical trials.
SYNTESIS. The original method for synthesizing fentanyl, patented by Paul Janssen in 1965, is a five-step process involving reductive amination, acylation, and amine deprotection/alkylation.[2] This method is suitable for pharmaceutical production but is too expensive and complex for large-scale production of chemical warfare agents. Below, under the spoiler, are synthesis methods developed in military or military-contracted chemical laboratories that can be used for pilot or industrial production of fentanyl group incapacitating agents.
Two key precursors for the synthesis of fentanyl and its analogs are N-phenethyl-4-piperidone (NPP) and 4-anilino-N-phenethylpiperidine (4-ANPP).
N-Phenethyl-4-piperidone (NPP).The starting materials for the most widely used method for the synthesis of fentanyl and its derivatives is N-phenethyl-4-piperidone (NPP) or 1-benzyl-4-piperidone. These compounds are obtained from the corresponding amine and methyl acrylate. The resulting diester is cyclized, hydrolyzed, and decarboxylated. The 3-methylfentanyl is prepared in a similar fashion — the amine first reacts with methyl methacrylate, followed by methyl acrylate.[50]

Dieckmann type condensation
R — –CH2Ph or –CH2CH2Ph (NPP)
This method was perfected by Iranian chemists involved in the development of non-lethal chemical weapons. The process allows for the production of NPP with nearly quantitative yields (97%) without distillation.[63]
Since the late 1980s, Lawrence Livermore National Laboratory (LLNL) has been involved in a program studying fentanyls as potential incapacitating agents. At LLNL, a method for producing fentanyl and thiofentanyl in excellent yields (73–78%) was developed. According to this method, the intermediate reagent NPP was obtained by reacting commercially available 4-piperidone with 2-(bromoethyl)benzene. At the next stage, NPP reacts with aniline, and the resulting product is treated with propanoyl chloride.[72]

Method Valdez
4-Anilino-N-phenethylpiperidine (4-ANPP). At Chemical Research, Development and Engineering Center (CRDEC), they worked on modifying two methods for synthesizing 4-ANPP:
- Reductive amination of NPP, also known as the Siegfried method, is named after the anonymous chemist who proposed it and published the procedure on the website www.rhodium.ws.[31] (shown in the top image). This method was later modified, including by chemists from the Chemistry and Chemical Engineering Research Center of Iran (CCERCI),[91–93], involved in a program for developing chemical weapons.[94,95] According to this method, fentanyl is obtained by reacting the intermediate imine with propionic anhydride, reducing the synthesis stage of 4-ANPP.[92]
- Reaction of NPP with aniline, KCN, and acetic acid followed by reduction with sodium borohydride (shown in the bottom image).[6] This synthesis method was developed by a group of researchers from CRDEC.[14] The intermediate α-aminonitrile can also be used for the synthesis of carfentanil.[19],

Methods Siegfried (at the top) and CRDEC (at the bottom)
The method for obtaining 4-ANPP from 4-anilinopyridine, developed by Lavrinovič from Latvian Institute of Organic Synthesis, does not require expensive reagents and is extremely simple. [62,70] Later, this method was significantly improved by Taiwanese chemists (Zee, 1980). The starting material, 4-anilinopyridine, can be obtained using Jerchel's method from aniline and pyridine. The overall yield (27%) of this process started from preparation of pyridylpyridinium chloride from pyridine is much better than any other known process.[71]

Method Lavrinovič-Zee
P. K. Gupta et al. (2010) from the Indian Defense Research and Development Organization (DRDO) developed a method for synthesizing 4-ANPP from 4-piperidone and aniline, followed by the reaction of 4-anilinopiperidine with 2-phenethyl bromide.[59]

Method Gupta (2010)
Additionally, the DRDO developed the One-Pot Synthesis of Fentanyl, which reduces the number of stages to three.[59]

Method Gupta (2005)
The whole reaction takes place under mild conditions and at room temperature.The process is low yielding (40%).
In the final stage of fentanyl synthesis, 4-ANPP is acylated with propionic anhydride or acetyl chloride.
The simplest and most accessible method for synthesizing fentanyl is the Gupta method. This method is used by chemists ("cooks") working for Mexican cartels to produce fentanyl. However, the fentanyl obtained by this method is heavily contaminated with impurities, unlike the fentanyl produced in China.[90]
α-Methylfentanyl
α-Methylfentanyl was first synthesized by Belgian chemist Paul Janssen in 1961[2], but it was only 30% stronger than fentanyl and did not attract much interest. As a result, the data on its analgesic activity were published much later, in 1974.[1]

α-Methylfentanyl
alpha-Methylfentanyl, China White, Persian White, 'glass'
(x1.3 fentanyl)[1]
In 1979, a new syntetic opioid called "China White" appeared on the black markets of California, causing numerous deaths. However, the dosage of the drug was so minuscule that the forensic analytical methods available at that time could detect nothing but sugar in the samples. In 1981, "China White" was mistakenly identified as 3-methylfentanyl, and it was only several years later that it was finally determined that an isomer of 3-methylfentanyl, α-methylfentanyl (AMF), was being sold illicitly under the "China White" label.[69]
In 1989–1990, the "China White" epidemic began to spread in the USSR. In clandestine laboratories in Leningrad, α-Methylfentanyl was synthesized by substituting phenethylamine with amphetamine in the standard fentanyl production method. Amphetamine was easier and cheaper to obtain than phenethylamine.
3-Methylfentanyl
The synthesis of genuine 3-methylfentanyl is much more complex, which is why it appeared on the illegal drug market later than alpha-methylfentanyl, in 1984. Along with it, more than 10 other illegal fentanyl analogs were The production of fentanyl and its derivatives is a highly profitable business. With precursor costs around $200, their street value can reach up to $2,000,000.[27]
3-Methylfentanyl is an extremely toxic substance with a lethal dose of less than 1 milligram. Its clandestine synthesis is deadly dangerous, with cases of poisoning among professional chemists even when protected by gas masks. Equally hazardous is the precursor phosgene (chemical warfare agent), which is used to remove the benzyl group instead of expensive palladium charcoal.[51]
In 1991–1992, clandestine production of 3-methylfentanyl began in Russia. It was synthesized by a group of talented students from universities in Moscow and Kazan, who already had experience in manufacturing Etorphine, Methadone, and its stronger analog Phenadoxone.[49] One of the precursors was methyl acrylate, which was used in the production of acrylic glass. This gave rise to the second popular street name for 3-methylfentanyl — 'glass'. Within a month, the underground chemists had synthesized 600 grams of the drug. The distribution of 3-methylfentanyl was handled by Kazan and Azerbaijani criminal groups.[37]
Not only in clandestine laboratories, but also at the Chemical Research, Development and Engineering Center (CRDEC), efforts were made to simplify and reduce the cost of fentanyl synthesis.[6] Furthermore, stereoselective synthesis allows for the production of more than a 13-fold excess of the more active cis-isomer.[3] A large number of 3-methylfentanyl analogs were synthesized and studied in the People's Republic of China (PRC).[73-79]
In 2002, during a hostage rescue operation in Moscow, where hostages had been taken by Chechen terrorists, an unknown narcotic gas was used, resulting in the deaths of 129 people. The theory that 3-methylfentanyl had been used was immediately supported by both foreign and Russian scientists, particularly from the Faculty of Chemistry of Moscow State University. It was only 10 years later that it became known that the "narcotic gas" contained not 3-methylfentanyl, but the more potent carfentanil and the faster-acting remifentanil.
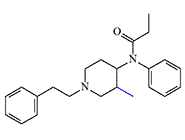
3-Methylfentanyl
Mefentanyl, 3-MF, F-7209, R-26 800, NIH 10456, MCV 4522
(x10-20 fentanyl)[27]
In animal experiments, 3-methylfentanyl was found to be 5 500 times more potent than morphine. In humans, analgesia and euphoria appear after taking only 0.005–0.01 mg, and a dose of just 0.3 mg can lead to respiratory arrest and death.[27]
Unlike fentanyl, which has no isomers, 3-methylfentanyl has two chiral centers in the molecule and four isomers that differ significantly in activity. Racemate of 3-methylfentanyl was first synthesized by T. Riley et al. (1973), the resulting drug was found to be 10 times more potent than fentanyl as an analgesic.[30] A year later, the most active isomer, (3R,4S)-cis-(+)-3-methylfentanyl, was synthesized in the laboratory of Janssen Pharmaceuticals.[1] This isomer is 19 times more potent as an analgesic than fentanyl.[35]
Besides the 3-methyl homologs, CRDEC showed interest in 3-oxy derivatives of fentanyl[26]. These substances are metabolites of fentanyls and were expected to have an improved pharmacological profile.[34] These expectations were partially met, as cis-(+)-3-methoxyfentanyl was found to be 28 times more potent as an analgesic than fentanyl,[48] but it did not find medical application.
 |
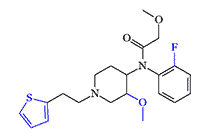 |
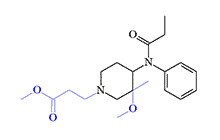 |
| 3-Methoxyfentanyl (x28 fentanyl)[48] |
Fentanyl BOC Group Inc. (x40 fentanyl)[26] |
Fentanyl Glaxo Inc. (x88 fentanyl)[11] |
Even greater opioid activity was found in the 3-methoxy-3-methyl derivative of phenylpiperidinylamide synthesized by Glaxo Inc., which turned out to be almost 90 times more potent than fentanyl.[11]
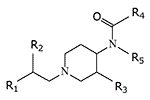 |
||||||||
| R1 | R2 | R3 | R4 | R5 | ED50 | Methods | Activity | Notes |
|---|---|---|---|---|---|---|---|---|
| Ph | H | Me | CH2CH3 | Ph | 0.0005 | HPsc | 1000 | NIH 10456[38] 3-MF |
| Ph | Me | Me | CH2CH3 | Ph | 0.00075 | TWiv | — | —[25] |
| 4-F-Ph | OH | Me | CH2CH3 | Ph | 0.00077 | HPsc | — | cis-FOMF[15] |
| Ph | OH | Me | CH2CH3 | Ph | 0.0001 | HPsc | 20000 -50000 |
NIH 10741 OMF[22] |
| Ph | OH | Me | CH2CH3 | 2-F-Ph | 0.00077 | HPsc | 6000 | NIH 10761[22] |
| Ph | OH | Me | CH2CH3 | 3-F-Ph | 0.001 | HPsc | 6000 | NIH 10784[23] |
| Ph | OH | Me | CH2OCH3 | Ph | 0.0004 | HPsc | 5000 | NIH 10719[24] |
| Ph | OH | Me | 2-furil | Ph | 0.0004 | HPsc | 6000 | NIH 10718[24] |
| Ph | OH | Me | CH2CH3 | 2-pyridinyl | 0.0002 | HPsc | 12000 | NIH 10726[24] |
| Ph | OH | Me | CH2CH3 | 4-Me-2-pyridinyl | 0.009 | HPsc | 12000 | NIH 10783[22] |
| Ph | H | OMe | CH2CH3 | Ph | 0.00064 | HPiv | — | —[26] |
| Ph | H | OMe | CH2OCH3 | 2-F-Ph | 0.00064 | HPiv | — | —[26] |
| 2-thienyl | H | OMe | CH2OCH3 | 2-F-Ph | 0.00046 | HPiv | — | —[26] |
| Ph | H | OMe | CH2CH3 | 2-F-Ph | 0.00091 | HPiv | — | —[26] |
| 2-thienyl | H | Me | CH2OCH3 | 2-F-Ph | 0.00056 | HPiv | 13000 | x29 fentanyl[29] |
| Ph | - | Me | CH2OCH3 | 2-F-Ph | 0.00069 | HPiv | — | x26 fentanyl[29] |
| COOMe | H | Me(OMe) | CH2CH3 | Ph | 0.000052 | TWiv | — | x88 fentanyl[11] |
TWiv — tail withdrawal test (rats, intravenous),
HPsc — hot plate test (mice, subcutaneous),
HPiv — hot plate test (mice, intravenous).
Activity — Activity was assessed by the ability to suppress withdrawal in morphine-dependent rhesus monkeys. The numbers indicate how many times the dose of the substance is smaller than the standard morphine dose of 3 mg/kg used to alleviate withdrawal symptoms.
In the early 1970s, Paul Janssen began studying a new group of analgesics from the series of 4-substituted fentanyls. In 1974, he synthesized carfentanil and sufentanil, and two years later, alfentanil. It was expected that these new compounds would be more effective and safer for humans, so the CRDEC prioritized them for further research.
In addition to the United States and Russia, research on potent fentanyl derivatives has been conducted in China, India, Iran, and other countries.
Research on Fentanyl Incapacitants in Other Countries
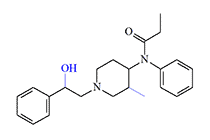
CHINA. Ohmefentanyl (OMF) was synthesized in China in the early 1970s, but the first article about it was only published in 1981.[44,77] The most active of its isomers (RTI-4614-4) is 6 300 times more potent than morphine and 28 times more potent than fentanyl in rodent experiments.[77] Ohmefentanyl shows even higher potency in primates: to alleviate withdrawal in monkeys, a dose 20,000–50,000 times smaller than that of morphine is sufficient.[16] However, ohmefentanyl has a lower potential for causing addiction compared to morphine,[39] which is likely why it has not yet appeared on the designer drug market.
In terms of analgesic potency, fentanyls are ranked in ascending order as follows: fentanyl < 3-methylfentanyl < ohmefentanyl in the ratio of 1:5:100 (rabbit). [73] Ohmefentanyl and Carfentanil are approximately equal in activity — x28 and x27 fentanyl, respectively (rats).[19]
 |
 |
| Ohmefentanyl (OMF, F-7302, F9204) (x28 fentanyl)[77] |
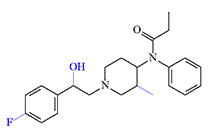
Fluoro-ohmefentanyl (4-FOMF) (x38 fentanyl)[15] |
Later, in 2002, Chinese chemists synthesized 4-fluoro-ohmefentanyl (4-FOMF), whose most active (3R,4S,2'S)-(+)-cis isomer is 1.37 times more potent as an analgesic than ohmefentanyl.[15]
Liquid analogs of 3-methylfentanyl. In the 1990s, interest in olefin derivatives of fentanyl grew in China, with some substances exhibiting remarkable biological activity. Experiments conducted at the Nanjing Military Hospital in 1992 showed that replacing the ethyl group in the 4-N-propionyl portion of 3-methylfentanyl with a chlorovinyl group increased analgesic activity by 1.5 times.[79]
 |
 |
| N-Chlorovinyl analog 3-methylfentanyl (x9 fentanyl)[77,79] |
Olefinic analogs of 3-methylfentanyl (x0.6–1.3 fentanyl)[78] |
All fentanyls known until the 1990s were crystalline substances, which complicated their military use. Attempts by Indian military chemists to employ thermal sublimation for dispersing fentanyls ended in failure. Liquid chemical warfare agents have several advantages: they can penetrate the skin, are easier to disperse using explosions, and can be used in binary munitions.[28]
In 1990, the Institute of Pharmaceutical Chemistry (Beijing) succeeded in obtaining liquid 1-β-substituted vinylethyl 3-methylfentanyls for the first time. As seen in the table, these substances surpass morphine in analgesic activity by several hundred times (See table).[78]
| Lab code | R | R' | R'' | Analgesic activity AD50 (mg/kg) |
Potency (Morphine=1) |
|---|---|---|---|---|---|
| 1309 | H | Me | cуclo-Pr | 0,106 | 131 |
| 1310 | Et | Me | Me | 0,0794 | 175 |
| 1312 | Et | Me | Cl | 0,05 | 277 |
| 1320 | Me | Cl | Cl | 0,047 | 293 |
| Fentanyl | 0.062 | 224 | |||
| Morphine | 13,9 | 1 | |||
Analgesic activity was evaluated using the "hot plate" test (mice, intraperitoneally).[77,78]
Liquid analogs of 3-methylfentanyl have high boiling points and low volatility, making it unlikely for their vapors to cause inhalation injuries. There is no information on how well liquid forms of fentanyl can penetrate the skin.
INDIA. Since the 2010s, the Defense Research and Development Organization (DRDO) has also shown increased interest in fentanyl derivatives. The compounds synthesized by Indian chemists are as active as fentanyl, but have a high safety index (See table). DRDO is also working on simplification and cheapening of methods fentanyl production, using so-called "one pot" synthesis, i.e. without isolation of intermediates.[59]
 |
 |
 |
| Fentanyl analogs developed by DRDO (Defense Research and Development Organization) (x1.2–1.4 fentanyl)[52,54] |
||
Vaporization of Fentanyls. Due to their crystalline state, most fentanyl-type incapacitating agents can only be applied in aerosol form. However, when fentanyl is administered as a coarse aerosol, doses nearly ten times higher than those required for intravenous administration are needed to achieve the same effect[45]. Only when using a nebulizer does the bioavailability increase to 100%.[8] Creating a finely dispersed aerosol cloud in an open space is a complex technical challenge. Indian chemists have attempted to use thermal sublimation to disperse fentanyl, as "production of smoke by heating is a low cost and convenient method that could generate respirable particles of compounds having significant military applications and other subversive use in civil population".[54]
| Compounds | R | Analgesic activity AD50 (mg/kg)* |
Potency (Fentanyl=1) |
Therapeutic index (LD50/AD50) |
Ref |
|---|---|---|---|---|---|
| Fentanyl | CH2CH2Ph | 0.028 | 1 | 625 | [52] |
| 1 | CH2CH2CH3 | 0.0267 | 1.05 | 5367 | [52] |
| 2 | CH2CH2OPh | 0.0237 | 1.18 | 4734 | [52] |
| 3 | CH2CH2CH2OPh | 0.0318 | 0.88 | 2843 | [52] |
| 4 | CH2CH2CN | 0.0333 | 0.84 | 8583 | [52] |
| 5 | CH2CH2NHPri | 0.0205 | 1.37 | 5551 | [53] |
| 6 | CH2CH2NHBut | 0.0210 | 1.33 | 5110 | [53] |
| 7 | -CH(CH3)COOPri | 0.0355 | 0.79 | 7828 | [53] |
| 8 | CH(CH3)COOBut | 0.055 | 0.51 | 6361 | [53] |
*Analgesic activity was assessed by the reaction to formalin injection into the hind paw of a mouse.
Preliminary experiments have shown that fentanyl is relatively stable when heated up to 350°C.[55] However, in studies on mice, the smoke of fentanyl and its derivatives (as shown in the figure above) was found to be extremely toxic to the animals, despite high therapeutic index of fentanyl analogs. This makes it unsuitable for use in pyrotechnic dispersal devices, unlike the incapacitant BZ or riot control agent CS. Additionally, smoke of fentanyl are not suitable for use as lethal chemical agents, as they are significantly less effective than the already known G- and V-series nerve agents.[54]
According to the authors of the experiment, "the study, however, concludes that due to possible decomposition of the compounds by heating or its poor absorption by the alveolar surface, the present inhalation technique cannot be employed to generate smoke of fentanyl and its analogs for any medical or surreptitious use."[54]
IRAN. According to the US ambassador to the OPCW, Iran is developing pharmaceutical-based agents (PBAs) for offensive or riot control purposes.[94] Iranian scientists do not conceal their research, periodically publishing articles about these agents in scientific and military-technical journals.
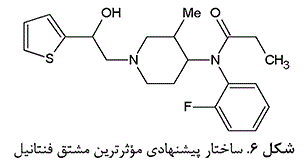 |
 |
| The presumed formula of the most active fentanyl (according to Iranian chemists) |
Thiofentanyl |
One of the leading centers for chemical weapons development is considered to be Imam Hossein University in Tehran. In 2008–2009, researchers from this institution published an article on the synthesis of fentanyl precursors.[63] In 2010, they published on the Structure-Activity Relationship (SAR) of 70 fentanyl analogs,[46] and in 2020, they reported on an improved method for the total synthesis of thiofentanyl.[64]
REPUBLIC OF SOUTH AFRICA. In the early 1990s, researchers from South Africa were searching for incapacitants from the fentanyl group. At least four fentanyl derivatives were synthesized and tested on mice without any success.[47]
Non-Opioid Anesthetics of the Fentanyl Series
Introducing an oxazoline ring into the fentanyl molecule completely removes its opioid properties but endows it with anesthetic properties. Compound A-3572 exhibits hypnotic effects comparable to those of diazepam, but with a shorter recovery time after awakening. Another interesting feature of this compound is its antagonism towards opioid-induced respiratory depression, while maintaining the level of analgesia.[32]
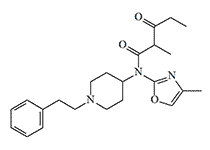 Compound A-3572 |
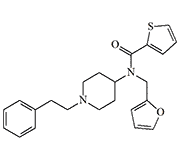
Compound Anaquest Inc |
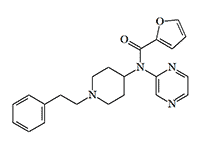
Mirfentanil |
The heterocyclic analogs of fentanyl synthesized by Anaquest Inc. are completely devoid of opioid activity. One of the most active compounds in this group immobilizes animals at the same dosage as fentanyl, but its effect lasts 4–5 times shorter.[32] The discovery of this group of anesthetics was enthusiastically received at the ERDEC,[33] as these substances prevent respiratory depression caused by opioids without affecting analgesia and anesthesia, unlike antagonists such as nalorphine.[36] Unfortunately, the most promising analgesic in this class, Mirfentanil (Mirfentanil), caused tachycardia and seizures in healthy volunteers at high doses.[43]