Toxicity of the azides of phosphoric acids
The azides of phosphoric and thiophosphoric acids were first synthesized and described in the early '50s and for the next several decades remained in the focus of attention of chemists involved in the development of two related groups of organophosphorus compounds — pesticides and poisoning agents. Their physiological effect is the same as that of G- and V-type chemical warfare agents and is manifested by inhibition of the enzyme cholinesterase.
The azide group N3 exhibits pseudohalogen properties, however, azides are less toxic than similar organophosphorus compounds containing fluorine. Nevertheless, military chemists hoped that the azides of phosphoric and thiophosphoric acids might have more preferable combat characteristics, such as resistance to hydrolysis, thermal stability, and optimal volatility. In addition, their synthesis did not require aggressive fluorine compounds.
In the USSR, phosphoric acids azides were studied at the Institute of Organoelement Compounds (INEOS). During the war and post-war years, research on second-generation organophosphorus CWAs such as sarin and soman was carried out under the guidance of Academician M. I. Kabachnik, and since 1959 the Institute participated in the secret state program on “Synthesis of new highly toxic substances, acting both through the respiratory tract and through the skin, in a series of organic phosphorus compounds containing sulfur, nitrogen, silicon, boron and other elements”[17]. In 1954 Prof. V. A. Gilyarov, the author of the most complete review on phosphoric acid azides[4], started working in Kabachnik's group.
Azidothiophosphinate, Azidothiophosphat and Methylphosphonoazidothioate
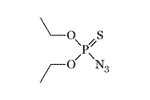
In the '50s, the renowned German chemist Gerhard Schrader synthesized Diethyl azidothiophosphate, a pesticide that is effective against insects but safe for warm-blooded animals[9]. As with other organophosphorus compounds, replacing two P-Alk bonds with two P-O-Alk bonds resulted in a significant increase in toxicity in Diethyl azidothiophosphate. This substance is slightly less toxic than tabun (GA), the German CWA synthesized before World War II[11]. Diethyl azidothiophosphate — is a colorless oil liquid, poorly soluble in water[7], and resistant to heating[12]. In the 60–70s, several publications on the synthesis of this substance were published in the USSR[12,14].
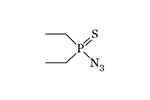 |
 |
|
| Diethyl azidothiophosphinate LD50 — 1000 mg/kg (rat, per os)[9] |
Diethyl azidothiophosphate LD50 — 5 mg/kg (rat, per os)[11] |
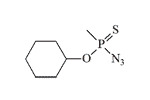
Azide analogs of thiosarin, thiosomane, and thiocyclosarin were synthesized at the U.S. Army Chemical Research and Development Laboratories between 1960 and 1961. The expectation was that “these azides were expected to be more volatile than V-agents and possibly retain the high mammalian toxicity of these agents.”[2] There are no published data on the physiological activity of these compounds.
 |
 |
 |
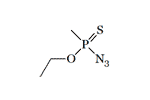 |
| Thiosarin azide | Thiosoman azide | Thiocyclosarin azide | O-Ethyl methylphosphonoazidothioate |
A report prepared by the Midwest Research Institute for the U.S. Arms Control and Disarmament Agency (ACDA) in the early '70s identified thiosarin azides as one of eight promising groups of chemical warfare agents (CWAs)[1].
In 1972, M. I. Kabachnik and V. A. Gilyarov published a method for the synthesis of O-Ethyl methylphosphonoazidothioate[18]. There is no doubt that other azides of thioanalogs of G-gases were obtained in the USSR.
Azidophosphate and Azidomethylphosphonate
Azidophosphate. In 1957, G. Schrader synthesized a structural analog of amiton, the first discovered chemical agent from the V-gas group, in which the diethylamine group was replaced by an azide group. The obtained substance was 3 times less toxic than amiton[8].
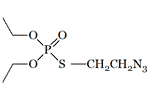 |
 |
 |
 |
| S-Azidoethyl O,O-diethyl phosphorothioate LD50 — 10 mg/kg (rat, per os) |
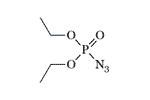
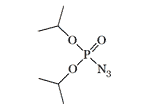
Diethyl azidophosphate | Diisopropyl azidophosphate LD50 — 2.5 mg/kg (mouse, i/p)[20] |
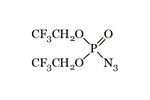
Bis(2,2,2-trifluoroethyl) azidophosphate |
Western[3] and Soviet[4] literature related to the development of chemical weapons mentions the “extremely high toxicity” and miotic action of diethyl azidophosphate. This substance is commercially available as it is used in peptide synthesis[12].
In the early '60s, V. A. Gilyarov synthesized an azide analog of the DFP (Diisopropyl fluorophosphate)[19], a chemical warfare agent manufactured by the British during the Second World War, which turned out to be even more toxic than the original[20]. In 1984, V. A. Gilyarov developed a method for the preparation of Bis(trimethylsilyl)azidophosphate, also similar in structure to DFP[16],
C. M. Timperley et al. (2001) from the British Defense Science and Technology Laboratory (Dstl) published a work on the synthesis of bis(2,2,2-trifluoroethyl)phosphorazidate[15]. Since no precautions for the extraction of this substance are mentioned in the article, it can be assumed that its toxicity is not higher than that of diethyl azidophosphate.
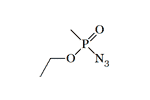
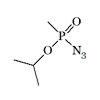
Azidomethylphosphonate. The use O-EthylSarin azide as a reagent is described by V. A. Gilyarov and M. I. Kabachnik in 1972[13]. It is very likely that other azides of G-gases were obtained by Soviet chemists. Sarin and its azide have similar toxicity in mice, 0.42 and 0.38 mg/kg (intraperitoneally)[20]. Probably high toxicity was found in structural analogs of V-type nerve agents containing an azide group, selenium, or silicon[20].
 |
 |
 |
 |
| O-EthylSarin azide | Sarin azide LD50 — 0.38 mg/kg (mouse, i/p)[20] |
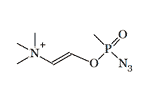
2-[Azido(methyl)phosphoryl]oxyvinyl-trimethylazanium LD50 — 0.36 mg/kg (mouse, i/p)[20] |
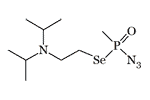
Azide Se-(2-diisopropylaminoethyl) methylphosponoselenoate (pI50 — 9.8)[20] |
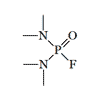
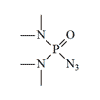
Azidomethylphosphonate with P-N bonds. Azides of phosphoric acids are not used in the agricultural industry. The only pesticide of this group, Mazidox, was on the market for a short time in the '50s[5]. As it turned out, it is very poisonous to humans and animals[6]. In chemical structure, Mazidox was an azide analog of another insecticide, Dimefox, in which the fluorine atom was replaced by an azide group. In the years of World War II, Dimefox was considered one of the most potent synthetic poisons.
 |
 |
 |
 |
| Dimefox LD50 — 1.2 mg/kg (mouse, i/p)[20] |
Mazidox (N.C.7) LD50 — 0.87 mg/kg (mouse, i/p)[20] |
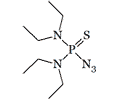
Bis(diethylamido) azidothiophosphate |
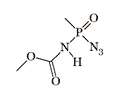
Methoxycarbonylamido methylphosphonoazidat LD50 — 1.2 mg/kg (mouse, i/p)[4] |
Extremely high toxicity has also been suggested for the thio-analog Mazidox[3]. The substances in this group have also been investigated at INEOS.[20].