Incapacitants of the Carfentanil Group

A lethal dose of carfentanil may not be visible to the naked eye (Photo DEA )
Fentanyl derivatives with a substituent in the 4-position of the piperidine belong to a separate group of incapacitants. These analgesics were synthesized much later than fentanyl and have a more attractive pharmacological profile, and three of them, sufentanil, alfentanil, and remifentanil have found medical applications. Carfentanil, which is 4-carbomethoxy-fentanyl by chemical structure, is used in veterinary medicine to immobilize large animals. The most famous substances of this group were synthesized and studied in the laboratory headed by Belgian pharmacologist Dr. Paul Janssen.
Carfentanil
Carfentanil is considered the strongest of the commercially available narcotic analgesics, being 40 times more effective than fentanyl and 8 000 to 10 000 times more effective than morphine. Apart from its extremely low effective dose, it has no advantages over other analgesics and is therefore only used in veterinary practice to temporarily immobilize large animals such as bears, rhinos, and elephants. Carfentanil is used in "durt guns" or in food baits, where it has replaced etorphine previously used for this purpose.
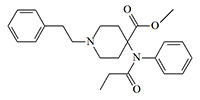
Carfentanil
Wildnil®, R33799 (citrate), R31833 (base), NIH 10570, Gray death (sleng)
Toxicity. The high therapeutic index of carfentanil in rodents and dogs led to the erroneous conclusion that in man the lethal dose would be hundreds and even thousands of times higher than the anesthetic dose. But, as it turned out, carfentanil, even in doses don't cause complete loss of consciousness, may cause fatal respiratory depression (see table).
| Dose (human, 70 kg) |
Route of administration |
Symptoms |
| 1,33 mcg (0.019 mcg/kg) |
intravenously | Dizziness (60%), nausea (33%), vomiting (6.7%), and itching (6.7%) (Minkowski, 2012).[62] |
| 2,1 mcg (0.03 mcg/kg) |
intravenously | Mild drowsiness (Newberg, 2009).[62] |
| 7 mcg (0.1 mcg/kg) |
intravenously | Mild ventilatory depression. (Frost, 2018).[63] |
| 8–15 mcg (0.1–0.2 mcg/kg) |
intramuscularly | Effective analgesic dose (Hess, 2018).[51] |
| 17.5 mcg (0.25 mcg/kg) |
intramuscularly | Euphoria. Sedative effect passing into sleep with vivid dreams. Analgesia. Reduction of the breath rate by two times. Moderate decrease in blood pressure and heart rate (Hess, 2018).[51] |
| 17,5 mcg (0.25 mcg/kg) |
intranasally | Pronounced analgesia and sedation 10 minutes after nasal administration of carfentanil solution (Hess, 2018).[51] |
| 24 0.34 mcg/kg |
intravenously | Predicted "severely toxic" dose (ECBC, 2018).[29] |
| 20–50 mcg (0.3–0.7 mcg/kg) |
intravenously | Minimal lethal dose (Stout, 2017,[48] Shafer, 2019).[63] |
| 50–100 mcg (0.7–1.4 mcg/kg) |
intravenously | Unconsciousness and severe respiratory depression (Hess, 2018).[51] |
Carfentanil is the most toxic substance synthesized by man, even more potent than chemical warfare agents such as VX and Novichok.[64] According to chemical toxicology analyses of fatal overdose victims, the lethal dose of carfentanil is about 50 mcg, and according to Houston Forensic Science Center's chief executive officer, Peter Stout, death can occur as early as 20 mcg (0.000 02 grams!) of the analgesic.[48] For comparison, the estimated median lethal dose (LD50) of the chemical warfare agent VX is about 600 micrograms per person.[26]
According to Major-General N. Antonov (1994), the median incapacitating concentration of carfentanil group analgesics for humans is estimated to be 0.07–0.35 mg·min/m3.[26] Calculations show that during the operation to free the hostages in the theater complex in Dubrovka (2002), it was enough to spray only 650 grams of carfentanil in the concert hall.[16] (see details)
Humans, monkeys, and pigs are all susceptible to respiratory depression after exposure to opioids.[43] In mice, rats and dogs, on the contrary, apnea occurs in doses thousands, and even tens of thousands of times higher than analgesic ones.
Rats. According to the Chemical Research, Development, and Engineering Center (CRDEC, 1989), the effective immobilizing dose (ID50) of carfentanil for rats is 31.9 mcg/kg for intraperitoneal administration and 3.7 mcg/kg for inhalation[14]. The median lethal dose for intravenous administration (LD50) is 3.39 mg/kg[59].
Ferrets. Median effective dose of ED50 (in mcg/kg, intraperitoneally) causing akinesia — 8.3, catalepsy — 18.2, loss of righting reflex — 37.9. The median lethal dose (LD50) is 83 mcg/kg. The Safety ratio (LD50/ED50) from 2.1 to 4.5 (CRDEC, 1989)[23]. When administered intravenously, the LD50 of carfentanil is 3 times lower at 27.77 mcg/kg, which "demonstrates the potency of carfentanil and its potential use as a terror agent." (USAMRICD, 2018)[54]
Rabbits. Intravenous administration of carfentanil at a dose of 0.34 mcg/kg induces loss of righting reflex, 1 mcg/kg causes collapse and prostration in 100% of cases. (ECBC, 2018) [29]
Dogs. For an unknown reason, dogs are more resistant to the effects of fentanyls than primates. Even doses of 100 mcg/kg carfentanil [52] and 3000 mcg/kg fentanyl[52] have no significant effect on the respiratory system.
Primates. A carfentanil dose of 2–4 mcg/kg causes severe respiratory depression in chimpanzees, and a dose of 14 mcg/kg in combination with 1.75 mg droperidol is used to euthanize the gorilla (Kearns K.S., 1999).[19]
In another experiment (Port J.D. et al., 1982), dogs and rhesus macaques were exposed to carfentanil aerosol in an isolation chamber. The concentration of the aerosol is not specified, but it is mentioned that the animals received 3.9 mg of carfentanil during a 5-minute exposure. Upon inhalation exposure to sprayed carfentanil, the animals consistently developed:
| after 2 min | Ataxia; |
| after 3 min | Leaning; |
| after 4 min | Сollapse; |
| after 10 min | Surgical analgesia; |
| after 30 min | Respiratory depression in monkeys. Animals exposed for 5 and 2 min were intubated 30 min after exposure and mechanically ventilated; |
| after 45 min | 50% of the dogs and the 15 and 30 sec exposure monkeys responded to painful stimulation; |
| after 60 min | All animals except the monkey exposed to carfentanil for 5 min responded to painful stimulation |
The conclusions of the experimenters: «Unfortunately, longer exposures than 30 sec in monkeys produced sufficient respiratory depression to necessitate mechanical assistance». This means that with an inhalation of approximately 20 mcg/kg and above, the rhesus monkeys stop breathing on their own. Dogs proved to be resistant to the effect of carfentanyl, and they did not require mechanical ventilation under similar conditions.[5].
In the next experiment (Port J.D. et al, 1989) by the same investigators, intravenous administration of carfentanil at a dose of 0.1 mcg/kg had no effect on rhesus macaques, and increasing the dose to 0.6–1 mcg/kg caused anesthesia and respiratory arrest, requiring administration of an antidote. The experimenters concluded that surgical levels of carfentanil induced anesthesia do not seem possible in the primate.[25].
A study conducted at the USAMRICD found that 0.71 mcg/kg of carfentanil subcutaneously induced bradypnea and/or loss of posture in African green monkeys in 50% of cases.[76]
Carfentanil and other ultra-potent opioids in solution form can penetrate through intact skin. With this method of administration, anesthesia in experimental animals occurred in 15–20 minutes.[60]
Synthesis. First synthesized in 1976 by Belgian pharmacologist Paul Janssen.[1,2] The first versions of the synthesis were so imperfect that in some steps the yield of the intermediate did not exceed 1%. In the early 90's for the needs of the U.S. Army were developed much more productive schemes for the preparation of carfentanil[3,6,55], which allowed to increase the yield of the final product from 1% to 34%.[78] Even more simplified the preparation of carfentanil proposed in 2010 simple and fast reaction Ugi-4CC, which allows in two stages to obtain remifentanil and carfentanil with a yield of 67–70%.[56] However, the starting material 1-isocyanocyclohexene can be stored indefinitely at -30 °C under an inert atmosphere, but darkens in a short time upon exposure to air.[39] The company selling chemical reagents offers it at a price of $350 for 50 milligrams. Yet, other modern methods of synthesizing carfentanil do not require expensive reagents and are relatively inexpensive.
In 2008, Iranian chemists at Imam Hossein University, known for their work on the synthesis of incapacitants and irritants, an efficient method was proposed for the preparation of phenethylpiperidone, the main precursor in fentanyl synthesis, from available and inexpensive phenylethylamine and methylacrylate.[68,69]
Alfentanil is a fast-acting but dangerous opioid
In 1987, the National Institute of Justice (NIJ) began funding the U.S. Army Chemical Research, Development, and Engineering Center (CRDEC) to search for and study potential chemical agents for temporary human immobilization.[45] The program was named Advanced Riot Control Agent Device (ARCAD).
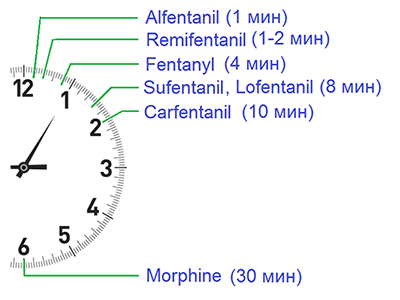
Time to onset of peak effect in rats, intravenous (Jansen, 1984)[40]
According to the developers, a precisely calculated dose of the drug was to be delivered to the target in a dart fired from a specialized weapon. The prototype of such a weapon was a standard police baton with a built-in dart gun with laser sights. Unlike aerosol, this method of delivery significantly reduced the risk of overdose and the development of side effects. However, this idea turned out to be unsuccessful. In 1989, the Lawrence Livermore National Laboratory joined the chemical incapacitant research with a $500.000 grant from the NIJ.[13]
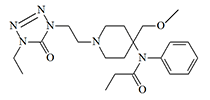
Given that the main requirement was rapid unconsciousness, the first candidate was the synthetic opioid Alfentanil (Alfenta®). In those years, alfentanil was considered the fastest-acting of all known opioids, its effects being felt as early as 20 seconds after administration. This analgesic was well studied and used in anesthesiology, which should have reassured public opinion. It is weaker than fentanyl, but its effect is much shorter — about 15 minutes. The first results of experiments with alfentanil were very encouraging, and in 1990, $580.000 were already spent on this research.[13]
 |
 |
| Alfentanil (Alfenta®) x0.03 carfentanil [71] |
|
However, exceeding the therapeutic dose only 4 times can lead to fatal respiratory arrest,[13] which is why researchers had to abandon further experiments with alfentanil and search for a safer alternative.
Unrivaled Power: Lofentanil, Ohlofentanil and Fluorocarfentanil
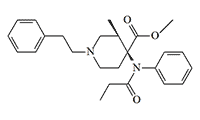
Lofentanil (3-Methylcarfentanil, R 34995). Disappointed with alfentanil, in 1993 the Livermore Laboratory scientists decided to switch to Lofentanil, which, although less fast-acting than its predecessor, was considered safer (at least for dogs). [22] Lofentanil is a (–)-cis-3-methyl derivative of carfentanil and is ten times more potent — in humans, analgesia and sedation occur after an intramuscular injection of 0.75 mcg (or 0.000 000 75 grams!). [73] Lofentanil is the most potent opioid ever tested in clinical trials.
Lofentanil is a leader among fentanyls in the duration of effect, which can last up to three days at high doses. Lofentanil forms a very stable complex with the μ-receptor, which makes overdose poorly amenable to treatment with the antidote naloxone. These properties made Lofentanil a bad analgesic, but a near-perfect murder weapon. This is probably why, in 1997, the Israeli Mossad chose Lofentanil to assassinate Khaled Mashal, the leader of the Hamas terrorist group. After Lofentanil was applied to Mashal's skin, the substance slowly penetrated the bloodstream, first causing drowsiness and dizziness, and then, after several hours, respiratory arrest. Attempts to neutralize the effects of the poison with naloxone failed. The only way to save Mashal was to put him on a ventilator until the effects of the drug wore off (see details).
 |
 |
 |
| Lofentanil x10 carfentanil[73] |
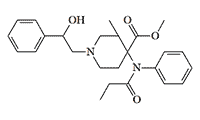
Ohlofentanil x1.3 carfentanil[74] |
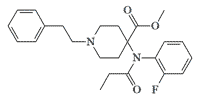
Fluorocarfentanil x7 carfentanil[28] |
Non-lethal weapons developers at Lawrence Livermore National Laboratory also refused to consider lofentanil as a potential incapacitant, due to its too high toxicity and lack of an antidote.[13] In addition, the sensitivity to the effects of the drug varied greatly between individuals.
Ohlofentanil (Ohmecarfentanil, RTI-4614-38). Also, as with ohmefentanil, a 3-methyl group in the piperidine and a 2-hydroxyl group in the phenylethyl chain increases activity by 20-33%.[46,74] Ohlofentanil and its ethyl ester, NIH 10792, are considered the strongest opioids tested in primates — in a test of withdrawal suppression in monkeys, these compounds are 30 000 times more potent than morphine.[70] The study of ohlofentanil and its derivatives was conducted at General Hospital of Nanjing Armed Forces (PRC).[74]
2-Fluorocarfentanil. In 1992, the Edgewood Research, Development and Engineering Center (ERDEC) achieved significant success in development of highly potent carfentanil derivatives with a high safety index. Researchers at ERDEC synthesized and studied 2-fluoroanilido derivatives of carfentanil, alfentanil, and sufentanil. Fluorocarfentanil showed 25% lower toxicity and 7 times higher activity than carfentanil in mice. Moreover, its safety index (LD50/ID50) was 8 times greater than that of carfentanil.[28]
One post at a forum about designer drugs describes a compound called 4"-Iodo-Ohmecarfentanil. A case was reported where the administration of just 3 micrograms (0.000 003 gram) of this substance nearly caused an overdose in a heroin addict with a high tolerance.[72]
Sufentanil — not very safe and slow-acting
In 1992, the US government stopped the Advanced Riot Control Agent Device (ARCAD) program due to the signing of an international treaty that prohibited the use of riot control agents as a form of warfare. However, research on derivatives of carfentanil at ERDEC and devices for their delivery at Lawrence Livermore National Laboratory continued. The primary focus of the research was on opioids that had an existing clinical database or FDA approval.[47]
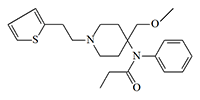
Sufentanil, first synthesized in 1974,[7] became the main candidate. Before the discovery of remifentanil, it was considered the safest opioid analgesic used in anesthesiology. When administered intravenously at a dose of 8 micrograms/kg and above, sufentanil causes sleep and anesthesia, but only with simultaneous inhalation of 100% oxygen.[57] The researchers suggested that using subanesthetic doses of sufentanyl in dart gun would be possible to immobilize a person without dangerous consequences.
 |
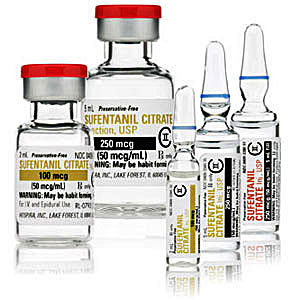 |
| Sufentanil (Sufenta®, R30730, R33800) x0.6 carfentanil[71] |
|
In 1995, experiments were conducted under the guidance of Prof. T. Stanley from the University of Utah on the use of sufentanil for human immobilization. T. Stanley is widely known as the inventor of the fentanyl lollipop for the treatment of chronic pain. He is also less known as the developer of non-lethal chemical agents for the fight against terrorism.
During the experiments, the volunteer was given an intramuscular injection of sufentanil at a dose of 1–3 mcg/kg and was instructed to walk around the room as long as possible. The results showed that at low doses of sufentanil, the volunteers lost the ability to move after 15 minutes, while at high doses, it took 10 minutes for the same effect to occur. However, even such low doses caused respiratory depression, which required the administration of an antidote.
In the early 2000s, T. Stanley continued the experiments by combining sufentanil with nalmefene. He believed that this combination of an opioid with an antagonist would accelerate the onset of the effect without causing respiratory arrest.[8,43]
Thiafentanil (Thianil, A-3080), an opioid similar in structure to sufentanil, is used in veterinary medicine as a safer alternative to carfentanil (therapeutic index x48 vs. x16 for carfentanil). To speed up the onset of immobilization in animals, veterinarians use hyaluronidase in the dart gun syringe. Thiafentanil may induce anesthesia within 2–3 minutes. Hyaluronidase can accelerate absorption and reduce induction times by 50%.[41]
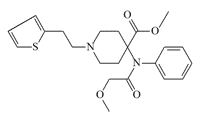
Thiafentanil (A-3080)
x0.7 carfentanil[65]
Experiments on immobilization of animals with thiafentanil and similar opioids were conducted at University of Utah School of Medicine in cooperation with the Chemical Research, Development and Engineering Center (CRDEC) in 1992.[61]
Remifentanil is a near-perfect analgesic
Remifentanil, a synthetic analgesic of the carfentanil group, was synthesized in 1980s by the chemists at Glaxo (and concurrently at a US Army laboratory)[20,71] and is currently available under the brand name Ultiva.
 |
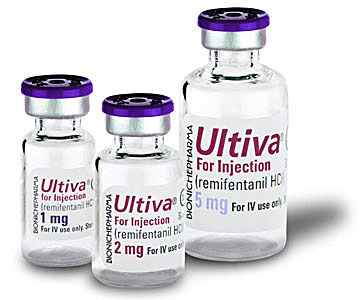 |
| Remifentanil (Ultiva®) | |
In 1989, US Department of Defense solicited commercial companies to participate in the development of less-than-lethal immobilizing chemicals: "Ideally one needs a material that will act safely, virtually instantaneously and last for just a few minutes. Thus, candidate chemical immobilizers with improved safety ratios and shorter duration of action are needed."[77]
Among commercially produced analgesics, only Remifentanil possesses the necessary properties. Remifentanil is metabolized rapidly in the human body by plasma esterases, which makes it the opioid with the shortest duration of action. After injection, the loss of consciousness is very quick, occurring 30 seconds later, and the drug's effect is terminated within 5–10 minutes.
By 1994, the basic research program at ERDEC had synthesized a series of short-acting fentanyls based on the Glaxo model and initiated preliminary toxicological studies.[21] In 2001, Edgewood Chemical Biological Center (ECBC, new name of ERDEC) evaluate the potential of the ultra-short acting anesthetic remifentanil as chemical immobilizing (incapacitating) agents for non-lethal applications for both military and law enforcement purposes.[15] Two years later, a mixture of remifentanil, carfentanil, and a chemical compound with a narcotic action (probably Halothane) was used Russian military to neutralize Chechen rebels in the Nord-Ost siege.[27] The victims of this "non-lethal chemical agent" were 132 hostages (see details).
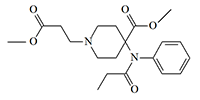
For many years, carfentanil was rightfully considered the most potent of the biologically active substances synthesized by man and was even included in the Guinness Book of Records. But in 1991, simultaneously with remifentanil, Glaxo synthesized an even more powerful analgesic from this group, exceeding carfentanil by 25 times. The chemical structure of this substance is remifentanil, in which the terminal methyl group is replaced by a tert-butyl group.
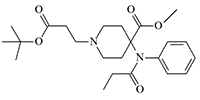
tert-Butyl homolog of Remifentanil
x25 carfentanil[20]
The effective analgesic dose of this substance in mice is only 0.021 mcg/kg versus 0.52 mcg/kg for the former leader carfentanil. The duration of analgesia is about 1.5 hours, so this substance is unsuitable for use as an incapacitant.[20] A brief mention of this opioid can be found in the monograph by Major-General of the Soviet Chemical Troops, N. Antonov, titled Chemical Weapons at the Turn of the Century.[26] So, it is very likely that fentanyls that has an immobilizing effect on humans at a dose of less than 1 microgram has already been developed today.
Czech researchers of incapacitating agents have investigated intranasal and conjunctival administration of remifentanil. These routes of administration may be useful for rapid analgesia in large numbers of casualties (disasters, accidents, etc.).[67]
Research on Carfentanyl Incapacitants in Other Countries


BBQ-901 anaesthetic gun system (PRC)
People's Republic of China. China is the only country that has produced a commercially available dart gun for human immobilization. The model "«BBQ-901 anesthetic gun system", for military and police use, allows to immobilize the enemy at a distance of up to 40 meters. According to the manufacturers, they managed to achieve an impressive fast action, after a hit "the target cannot move and counteract for less than 1 minute."[30]
According to researchers at the British Defence Science and Technology Laboratory (Dstl), vinyl-carfentanil is "relevant to defence and security."[36] Vinyl-carfentanil (Compound 1385), synthesized in 1990 at the Beijing Institute of Pharmaceutical Chemistry, is approximately equal to carfentanil in activity, but its two analogues numbered 1379 and 1387 are 1.6 and 2 times more potent than carfentanil, respectively.[9]
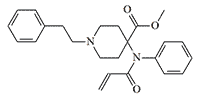 |
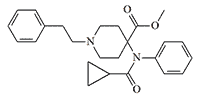 |
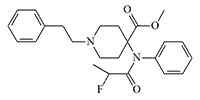 |
| Vinyl-carfentanil x1.2 carfentanil |
Compound 1379 x1.6 carfentanil |
Compound 1387 x2 carfentanil |
In 1994, Chinese authors published toxicological data on several olefinic derivatives of carfentanil, which exhibited unusually high safety ratios for this group. In the selected experimental animal, rabbits, the pharmacokinetic profile of carfentanil is similar to that in humans, which means the difference between the lethal and immobilizing doses is small (unlike in rodents).[29] For the 3-butenyl derivative of carfentanil, the dose causing anesthesia was 112 times lower than the lethal dose.[79] Among the synthesized fentanyls, there were some that exceeded the toxicity of the chemical warfare agent VX, which has an LD50 of 0.0084 mg/kg.[42]
 |
 |
 |
| Anesth. ED50 — 0.018 mg/kg LD50 — 2,0 mg/kg |
LD50 — 0,008 mg/kg | Compound 1302 LD50 — 0,005 mg/kg |
Studies on the activity and synthesis of carfentanil, ohlofentanil, lofentanil, and their numerous derivatives were carried out with the participation of the Hospital of Nanjing Armed Forces in 1992–1994[74,75].
ČSSR and the Czech Republic. In 1985, a Czechoslovakian physician L. Hess received 1 gram of the new powerful analgesic carfentanil from Janssen Pharmaceutica. The drug was tested on three nurse volunteers. At a dose of 0.25 mcg/kg, carfentanil produced analgesia, sedation, and sleep when administered intramuscularly and intranasally. However, even this "homeopathic" dose decreased the respiratory rate by half.[51]
Until 1989, the Czechoslovak Socialist Republic (ČSSR) was a member of the Warsaw Treaty Organization and worked with the USSR to develop new types of non-lethal chemical weapons. After the collapse of the ČSSR and its accession to NATO, after a long hiatus, the Czech Republic resumed work on "pharmacological non-lethal weapons" in the early 2000s. In 2005, at the 3rd European Symposium on Non-Lethal Weapons, a group of Czech scientists presented a paper on the inhalation effects on rats of various formulations, including those based on remifentanil, sufentanil, and alfentanil.[31]
According to the data obtained by Czech scientists, ultra-potent opioids such as carfentanil and sufentanil completely suppress aggression in experimental animals, which is a very important quality for chemical incapacitants.[8]

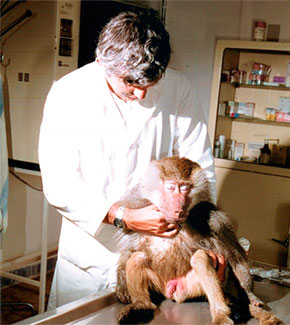
Lack of aggressive response in the baboon (left) and rhesus macaque (right) after carfentanil administration. (L. Hess, 2018)
Soviet Union / Russian Federation. The Soviet Union, and later the Russian Federation, have extensive experience not only in obtaining ultra-potent fentanyls, but also experience in combat and counter-terrorism use. In addition to the already mentioned carfentanil and remifentanil, researchers from the Defense Science and Technology Laboratory (Dstl) suggest that another of the potential incapacitant of this group could be a substance — a hybrid of carfentanil and the hypnotic drug Clomethiazole.[27]
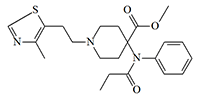
4-Methylthiazole analog of carfentanil
x0.6 carfentanil
The assumption is based on a publication on the laboratory synthesis of Clomethiazole, which has among its authors Russian chemists who were involved in military research on fentanyl.[37] One of the authors of the publication, Artur Zhirov, is the former director of the 27th Scientific Center and is under US and European Union sanctions for his involvement in the poisoning of Aleksey Navalny.[35]
This substance can likely be obtained using the method developed at the Edgewood Chemical Biological Center (ECBC) in a reaction between Clomethiazole and Norcarfentanil.[34,55] This substance is only 1.5 times less active than carfentanyl,[38] but as experiments by Chinese researchers have shown, replacing the phenyl group with thiazole significantly increases the safety ratios (LD50/ED50).[79]
One of the former employees of GosNIIOKhT (State Scientific Research Institute of Organic Chemistry and Technology) mentioned that the institute was developing a binary chemical weapon based on fentanyls.[50]
USA. Experiments on the effects of carfentanyl aerosol on primates have been conducted at the Edgewood Research, Development and Engineering Center (ERDEC) since the 1980s, However, the results have never been published [58], because most documents on the incapacitants of this group are still classified as "secret". For example, one of the works published by ERDEC in 1992 provides data on the activity of more than 60 fentanyl derivatives, but some information on the most active compounds is missing from the publication.[49]
In the early 1990s, ERDEC attempted to create carfentanyl derivatives that would have the properties of both an agonist and an antagonist for opioid receptors. These drugs, such as naltrexone and nalorphine, have a relatively high safety ratios for opiate use, meaning they rarely cause fatal overdoses.
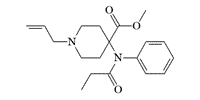 |
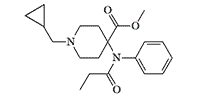 |
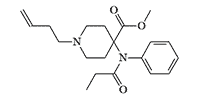
N-Allyl analog of carfentanil |
N-Cyclopropylmethylene analog of carfentanil |
However, carfentanil analogs, with N-allyl and N-cyclopropylmethylene groups typical of opioid agonists/antagonists, "exhibited safety ratios (LD50/MED50) in the usual range of fentanyls."[78]