Development of Blister Agents in the First Half of the 20th Century
Aliphatic Nitrosocarbamates
During World War II, scientists from the U.S. National Defense Research Committee (NDRC), who were involved in the development of chemical weapons, took an interest in a compound called N-methyl-N-nitrosocarbamate. This substance was used in the chemical industry and often caused severe skin damage to workers who came into contact with it. However, it did not have the level of toxicity necessary to be considered a chemical warfare agent. Using its molecule as a basis, researchers synthesized and conducted toxicological studies on 35 nitrosocarbamates. Among these, two compounds, designated KB-10 and KB-16, were of particular interest. These substances caused severe irritation of the eyes and skin in humans, and exposure to high concentrations led to pulmonary edema, coma, and death. In experiments on mice, KB-16 was found to be as toxic as mustard gas — the "king of gases" from World War I—and was even more toxic when absorbed through the skin. However, the main advantage of KB-16 over mustard gas was its lack of a pronounced odor. In fact, its pleasant fruity scent was only detectable at concentrations ten times higher than that of mustard gas.[1]
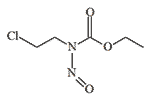 |
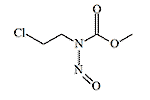 |
| KB-10 | KB-16 |
In 1942, the head of the NDRC informed his British counterpart that tests on KB-16 had shown a "high degree of toxicity" and that a concentration of 30 mg/mм3 would incapacitate victims and produce death within ten days. [5] Soon after, the Americans regretted their candor, as they wanted the development of the new, promising chemical warfare agent to be conducted exclusively by the U.S. and under strict secrecy. Fearing that information might leak to the enemy, they discouraged their allies from participating in the project under various pretexts, arguing that the British lacked sufficient capacity to produce the necessary raw materials and that the Canadians should avoid conducting field tests on KB-16 due to its instability.[5] Meanwhile, the American company DuPont was planning to manufacture up to 500 tons of KB-16 per month.[1,6]
KB-16 (N-(β-chloroethyl)-N-nitrosomethylcarbamate, TL-186, T1792) is an orange-red transparent liquid, poorly soluble in water. It is thermally unstable, breaking down when heated or subjected to an explosion. The production of KB-16 was more complex than that of mustard gas and lewisite, but the raw materials were inexpensive and readily available. Its inhalation toxicity is lower than that of mustard gas due to its low volatility of 870 mg/m3, but KB-16 solidifies at a lower temperature, making it potentially suitable for use in winter conditions. In 1942, tests on a formulation known as HQ (80% mustard gas and 20% KB-16) were conducted on volunteers at the Chemical Defence Experimental Station (CDES) in Porton Down, where KB-16 likely served as an antifreeze agent.[4]
In 1942, the USA and the U.K. began a more thorough study of KB-16 and discovered several significant drawbacks. In larger animals (dogs, goats, sheep, and monkeys), the toxicity of KB-16 was found to be lower than that of sulfur mustard (H) and nitrogen mustard (HN3). During storage, KB-16 quickly lost its potency, and all attempts to find a suitable stabilizer ended in failure. Additionally, its main competitor as a mustard gas replacement — nitrogen mustard (HN3) — was just as toxic, more stable, and also nearly odorless.[1]
KB-16 possesses insufficient storage stability to be seriously considered for large-scale manufacture for chemical warfare purposes. To address this issue, a special "binary munition" was proposed. In such a shell, methyl N-(β-chloroethyl)carbamate and nitrous gases were separated by a partition. Before firing, or even thereafter, the partition would break, allowing the components to mix and react, forming KB-16. However, even with these measures, it was not possible to improve the combat effectiveness of nitrosocarbamates — they still degraded rapidly in the field, and the reaction between the original substances produced a low yield of the final product.[1]
Research on nitrosocarbamates in the U.S. continued into the post-war period, as some of them were found to have anti-cancer properties. In 1957, the Chemical Warfare Laboratories, in collaboration with medical centers, published a paper on the toxicity and anti-cancer activity of KB-16 derivatives. One of these compounds, N-nitroso-N-(2-chloroethyl)propionamide, exhibited a unique property. This compound that gave evidence of decomposition on storage, became increasingly toxic and finally attained a toxicity higher than that of nitrogen mustard (HN2).[2]
In the 1940s, the synthesis of vesicants from the nitrosocarbamate group was undertaken by Nazi chemist H. Brintzinger, known for his work in the field of Wehrchemie (military chemistry, specifically the military use of poison gases).[3]
Derivatives of Acetylene and Ethylene
Dimethyl acetylenedlcarboxylate (DMAD) H3COOC–C≡C–COOCH3 is a potent lacrimator and exhibits rather unusual vesicant properties. Two days after even a few drops of DMAD contact the skin, a painless burn appears suddenly, often manifesting as blisters or even necrosis. Full healing can take several weeks. The substance can penetrate protective clothing, and burns can be caused by just a few drops of a 1% solution. At such low concentrations, DMAD is odorless, making this chemical agent even more dangerous.[2]
First synthesized in 1882,[3] this substance has attracted the attention of military chemists for decades. In the 1920s, French chemist Charles Moureu, the godfather of acrolein[4], worked with it. During World War II, a group led by Carl R. Noller at Stanford University, which was searching for new promising chemical warfare agents, synthesized and tested 33 derivatives of acetylene and ethylene.[5] The most toxic of the synthesized compounds, 1-methoxy-2-pentyn-4-one H3C–C(O)–C≡C–OCH3, had a median lethal concentration (LC50) of 600 mg/m3, while eight other compounds had LC50 values below 3000 mg/m3.[9]
Formula |
Action |
Ref |
|---|---|---|
| Cl–CH2–C≡C–CH2–OH | Powerful skin irritant | [1] |
| Br–CH2–C≡C–CH2–OH | Strong skin irritant | [1] |
| Br–CH2–C≡C–CH2–Cl | Powerful skin irritant and a lachrymator | [1] |
| I–CH2–C≡C–CH2–Cl | Vesicant and a lachrymator | [1] |
| HC–C≡C–COOCH3 | Caused intense lacrimation | [9] |
| HC–C≡C–COOC2H5 | Caused intense lacrimation | [9] |
| HC–C≡C–COOC2H4Cl | Caused intense lacrimation | [9] |
| HC–C≡C–COOH | Vesicant on application 100 mcg | [9] |
| I –C≡C–CH2–OH | Vesicant on application 100 mcg | [9] |
| HC≡C–CN | Its vapor irritates mucous membranes | [11] |
| C6H5–C≡C–CN | Its vapors are extremely irritating to the eyes | [12] |
Structurally similar to DMAD, diethyl fumarate H5C2OOC–CH=CH–COOC2H5, both in liquid and vapor forms, often causes redness and itching of the skin. These symptoms usually disappear after a few hours[2]. In 1944–1945, a series of esters and halogen derivatives of fumaric acid were synthesized and tested on volunteers at Stanford University. These experiments were part of the U.S. chemical weapons rearmament program.[9]
Fumaric acid nitrile CN-CH=CH-CN, in vapor and fine particle forms, causes irritation of mucous membranes and has both blistering and lacrimatory effects.[1] In 1971, the British Chemical Defence Experimental Establishment (CDEE) published the results of testing fumarodinitrile on volunteers. It was found to be five times less potent as a vesicant compared to sulfur mustard (H).[10]
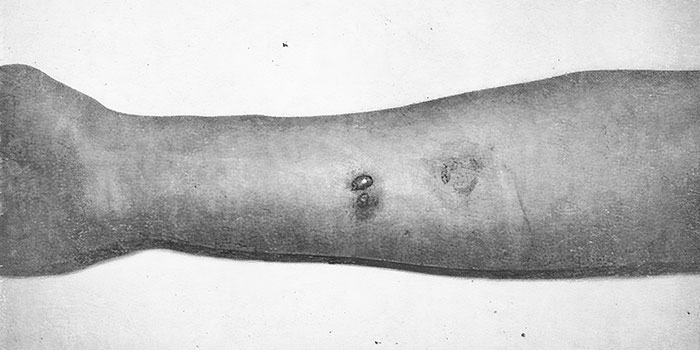
Effect of fumarodinitrile solution on human skin. Left — vesicles after applying 0.2 mg; right — erythema after 0.1 mg (Holland, White, 1971).
2,3-Butadiene-1-ol (H2C=C=CH–CH2–OH) is a colorless liquid with a boiling point of 126–128°C. It exhibits powerful vesicant effects on the skin and causes irritation of mucous membranes[8]. In 1933, it was tested as a chemical warfare agent at the Edgewood Arsenal but was deemed unsuitable.[7]
Pyrimidine derivatives
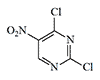
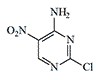
Pyrimidine derivatives represent another little-known group of experimental blister agents that were unsuitable for military use but caused significant discomfort to personnel in various chemical laboratories. In the mid-1920s, the Royal Engineers Experimental Station (United Kingdom) conducted tests on 2,6-dichloro-5-nitropyrimidine (T.348) and 2-chloro-6-amino-5-nitropyrimidine (T.349) as potential chemical warfare agents.
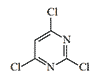
2,4,6-Trichloropyrimidine is a volatile liquid that crystallizes at room temperature and acts as both a lacrimator and a pulmonary irritant. When it comes into contact with the skin, either in liquid or vapor form, it causes a burning sensation, followed by blister formation. Skin lesions may take up to two days to appear after contact. Rubber gloves do not always provide protection against this vesicant.[1]
 |
 |
 |
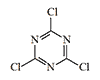 |
| T.348 | T.349 | 2,4,6-Trichloropyrimidine | Cyanuric chloride |
Widely used in organic synthesis, cyanuric chloride also strongly irritates the mucous membranes of the eyes and nose. The minimum irritating concentration is just 0.3 mg·min/m3. There is a reported case of a rash developing after exposure to a negligible amount of cyanuric chloride vapors during routine weighing in a laboratory. There is no doubt that closer contact would result in severe skin damage.[1]
Phenylenediamines
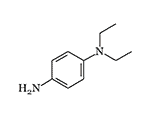
N,N-Diethyl-p-phenylenediamine and its analogs have been used in analytical chemistry, photography, and dye production for over a century, continuing to pose significant challenges to chemists working with them.[1,2] The toxic effects of dimethyl- and diethyl-p-phenylenediamine were first thoroughly documented in 1923 by U.S. Chemical Warfare Service Captain Paul J. Hanzlik. Both substances caused pain, redness, swelling, and rash when applied to human skin in amounts as small as 0.001 ml, with higher doses leading to blister formation.[5] However, these p-phenylenediamine derivatives were entirely unsuitable as chemical weapons, as they rapidly degraded even at room temperature.
In 1944, Smit and Engelhardt, who were involved in the search for new chemical warfare agents for the U.S. Army, synthesized and studied the toxicity of 43 p-phenylenediamine derivatives. These substances were of interest as potential blister agents. The most toxic tetraethyl derivative had a minimum lethal dose of 10 mg/kg,[3] which is comparable to the toxicity of a well-known poison like potassium cyanide.
 |
 |
| N,N-Diethyl-p-phenylenediamine | N,N,N',N'-Tetraethyl-p-phenylenediamine |
In 1955, under a contract from the CIA, one of its chemical contractors synthesized about 100 grams of N,N-dimethyl-p-phenylenediamine for use in a project under the MKULTRA program[4]. This substance is extremely toxic and easily penetrates the skin. Hanzlik (1923) noted that applying just 3.55 ml to the skin is sufficient to kill an adult human.[5]